Abstract
Purpose
Oral human papillomavirus (HPV) infection is a risk factor for head and neck squamous cell carcinoma (HNSCC), and is a concern for patients with HPV-positive HNSCC and their partners. The prevalence of oral HPV infection before and after cancer therapy was investigated among patients with HPV16-positive and -negative HNSCC.
Experimental design
Serial oral rinse samples (ORS) were collected from a cohort of 135 HNSCC cases as frequently as every three months for up to three years. Tumor HPV status was determined by HPV16 in situ hybridization. HPV was detected in ORS by consensus PCR and line-blot hybridization. The HPV16 variants in positive oral rinse-tumor pairs were determined by sequencing. The odds of oral HPV infection among HPV16-positive and -negative cases were compared by use of generalized estimating equations.
Results
Patients were followed for a median of 21 months and provided a median of four samples. Forty-four of 135 patients had HPV16-positive tumors. HPV16-positive cases were more likely than HPV16-negative cases to have an oral HPV infection detected before (OR=8.6, 95%CI=3.5–21) and after therapy (OR=2.9, 95%CI=1.1–7.4). Oral infections by HPV16 and other high-risk, but not low-risk, types were more common among HPV16-positive cases both before and after therapy. Most HPV16 variants in ORS were European, unique, and identical to that in the tumor. Persistence of a type-specific oral infection was demonstrable for as long as five years.
Conclusion
Oral high-risk HPV infections are more frequent among patients with HPV16-positive than HPV16-negative HNSCC, consistent with a behavioral and/or biological disposition to infection.
Keywords: human papillomavirus, oral infection, head and neck cancer
Introduction
Case-control studies have now established that oral HPV infection is a strong risk factor for head and neck squamous cell carcinoma (HNSCC), and particularly for oropharyngeal cancer. High-risk oral HPV infection, but not low-risk infection, is strongly associated with cancer risk. (1–3) Risk is particularly high for oral HPV16 infection. Oral HPV16 infection was independently associated with a 15-fold increase in risk for oropharyngeal cancer in a recent case-control study, (4) a risk estimate similar to the 14-fold increase in subsequent risk for oropharyngeal cancer among HPV16 seropositive individuals in a nested case-control study (5).
Oral HPV infection therefore has important health consequences, and yet, little is known about risk factors for infection. Limited available data suggest that oral HPV infection is likely sexually acquired: a history of sexually transmitted disease and number of oral sexual partners are associated with both oral HPV infection (6, 7) and HPV-positive oropharyngeal cancer. (4) Additionally, the presence and persistence of an oral high-risk HPV infection has been associated with a persistent oral infection in a spouse (8). Consistent with this data are the increased risk for oral cancer among husbands of women with cervical cancer (9, 10) as well as the increased risk for oral cancer among women with a history of cervical cancer. (11, 12) Indeed, husband-wife pairs have been diagnosed with HPV16-positive tonsillar cancer and viral sequencing revealed the HPV16 variant to be identical in the tumors, consistent with transmission of the virus between the couple. (13)
The association of oral HPV infection and oral cancer is therefore of particular concern for partners of patients diagnosed with HPV-positive HNSCC. Patients and their partners frequently seek guidance from physicians as to whether patients with HPV-positive HNSCC are potentially infectious before or after treatment for their cancer. As a first attempt to address this issue, we evaluated whether patients with HPV-positive HNSCC were more likely than patients with HPV-negative HNSCC to have a detectable oral HPV infection before or after cancer treatment. We present these preliminary data in an effort to provide some evidence-based guidance for clinicians and their patients.
Materials and Methods
Patient population
The study population consisted of patients who were enrolled in a longitudinal cohort study designed to evaluate molecular-genetic indicators of cancer recurrence in oral rinse samples. Eligible cases included patients over the age of 17 years who were diagnosed with histopathologically confirmed, head and neck squamous cell carcinoma at the Johns Hopkins Hospital between 2000 and 2006. The protocol was approved by the Institutional Review Board of the Johns Hopkins Hospital and written informed consent was obtained from all participants.
Oral rinse samples were collected via a 30 second saline oral rinse and gargle prior to initiation of cancer therapy and as frequently as every three months after therapy for up to three years. Paraffin-embedded tumor samples and in a subset of cases the corresponding fresh-frozen tumor sample were collected from the diagnostic biopsy for determination of tumor HPV status.
Patients were prospectively followed for clinical evidence of cancer recurrence by physical examination and diagnostic imaging studies per the standard of care. All laboratory analyses were performed masked to clinical data and tumor HPV status.
Tumor HPV status
Cases were classified as diagnosed with an HPV-positive or negative tumor based upon the results of HPV16 in situ hybridization (ISH). Analysis was performed on the primary tumor except for in the case of an unknown primary when the nodal disease was evaluated. HPV16 was detected in paraffin-embedded tumor samples by use of the ISH catalyzed signal amplification method for biotinylated probes (Dako GenPoint, Carpinteria, CA) as previously described.(14) Cases were considered positive for HPV16 if a punctate signal specific to tumor cell nuclei was detected.
For a subset of the HPV16-positive tumors, tumor DNA was purified from fresh-frozen tumor samples or from paraffin-embedded tumor samples via proteinase K digestion, phenol-chloroform extraction and ethanol precipitation.
Oral HPV infection detection
Oral rinse sample processing and pre-amplification assay set up was performed in dedicated laminar flow hoods in a specimen processing laboratory separate from the post-amplification analysis laboratory. DNA was purified from saline oral rinses by proteinase K digestion, phenol chloroform extraction and ethanol precipitation as previously described (15). As a control for inter-specimen contamination during DNA purification, a single aliquot of an HPV-negative cell line control (K562, 105 cells per ml saline) was processed per 29 oral rinse samples.
Purified DNA was analyzed for 37 HPV types by multiplex polymerase chain reaction (PCR) targeted to the L1 region of the viral genome using PGMY09/11 L1 primer pools and primers for β-globin, followed by hybridization to a linear probe array (Roche Molecular Systems, Inc., Alameda, California). (16, 17) Samples were tested in 96-well plate format with a negative control included in each row of the plate (per 11 oral rinse samples). Samples that were β-globin negative were considered to be of insufficient quality for analysis. Samples were reported as positive or negative for HPV genomic DNA and the HPV type was reported for positive samples.
HPV16 viral copy number was measured in purified DNA from oral rinse samples by use of a validated, real-time, TaqMan PCR method targeted to the E6 coding region of the viral genome (18). Samples with ≥1 viral copy were considered positive. Positive samples were retested in duplicate and viral load was reported as the mean of the duplicates. Measured HPV16 viral load was adjusted to an estimate of the number of cells in the sample as measured by real-time PCR targeted to a single copy human gene, human endogenous retrovirus 3 (ERV-3) (15).
HPV16 variant analysis was performed for HPV16-positive oral rinse samples and corresponding HPV16-positive tumor samples, if available, via PCR amplification of the E6 coding region, base pairs 31–640 (GenBank NC 001526)(19). PCR products were purified from unincorporated nucleotides and primers by adding ExoSAP-it (USB, Cleveland, OH) for 15 min at 37 °C and then at 80 °C for 15 min. Fluorescent tagged dideoxy sequencing reactions were performed using Big Dye v. 3.1 (Applied Biosystems, Applera Corp., Foster City, CA) for 30 cycles and purified from unincorporated fluorescent nucleotides using a 96 well Millipore Millex plate packed with Sephadex G50 (GE Healthcare, Piscataway, NJ). Sequencing reaction products were dried in a sample concentrator (Appropriate Technical Resources, Laurel, MD), resuspended in Hi-Di formamide (ABI) containing 1 micromolar EDTA, and loaded onto a 96 well thermal gradient capillary electrophoresis instrument (Spectrumedix/Transgenomic, Omaha, NE). At least two sequencing reads were performed for each variant. Amplification and sequencing was repeated to resolve discrepant results. Sequence traces were generated and analyzed using BaseSpectrum (Spectrumedix/Transgenomic) and Sequencher software (Gene Codes, Ann Arbor, MI), and were compared to the HPV16 European reference sequence (GenBank, NC 001526) and categorized by variant as previously reported (20).
Statistical analysis
Summary statistics used to describe the data include proportions for categorical or ordinal variables and median and interquartile range (IQR) for continuous variables. The characteristics of the HPV-positive and negative patients were compared by use of Fisher’s exact test. Generalized estimating equations were used to model the odds of oral HPV infection among HPV-positive and HPV-negative head and neck cancer cases while accounting for correlated data arising from repeated measures within an individual. Values were adjusted for age, gender and race based on prior associations with oral HPV infection (7). Associations were considered statistically significant for a two-sided p≤0.05. SAS 9.1 (SAS Institute, Cary, NC) was used for all analyses.
Results
Characteristics of the study population
The study population consisted of 135 patients with newly diagnosed squamous cell carcinoma of the oral cavity, pharynx, larynx, sinus, or unknown primary (Table 1). Median age at diagnosis was 57 years (range 25 to 82) and 77% were men. The majority of patients (n=118) received primary surgical therapy with or without adjuvant radiotherapy.
Table 1.
Demographic characteristics, tobacco and alcohol consumption, tumor characteristics and treatment modality among HNSCC patients (N=135)
| Explanatory Variables | HPV16 positive N=44 (%) | HPV16 negative N=91 (%) | Fisher’s exact test p-value |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Gender | 0.002 | ||
| Female | 3 (7) | 28 (31) | |
| Male | 41 (91) | 63 (69) | |
| Age | 0.02 | ||
| <50 | 14 (32) | 24 (26) | |
| 50–64 | 26 (59) | 40 (44) | |
| ≥ 65 | 4 (9) | 27 (30) | |
| Race and ethnicity | 0.01 | ||
| White, non-Hispanic | 43 (98) | 75 (82) | |
| Black, non-Hispanic | 0 (0) | 13 (14) | |
| Other | 1 (2) | 3 (3) | |
| SMOKINGa | |||
| Pack-years smoked (cigarettes, cigar or pipe) | 0.002 | ||
| Non-smoker | 24 (55) | 27 (30) | |
| 1 to 19 | 9 (20) | 9 (10) | |
| ≥ 20 | 8 (18) | 36 (40) | |
| Unknown | 3 (7) | 19 (21) | |
| DRINKINGa | 0.12 | ||
| Number of years drank ≥ 15 drinks per Week | |||
| Non-drinker | 29 (66) | 44 (48) | |
| 1 to 14 | 4 (9) | 7 (8) | |
| ≥ 15 | 9 (18) | 21 (23) | |
| Unknown | 3 (7) | 19 (21) | |
| TUMOR CHARACTERISTICS | |||
| AJCC TNM stage | <0.0001 | ||
| Stage 0 | 1 (2) | 3 (3) | |
| Stage I | 1 (2) | 34 (37) | |
| Stage II | 2 (5) | 19 (21) | |
| Stage III | 4 (9) | 14 (15) | |
| Stage IV | 36 (82) | 21 (23) | |
| Tumor anatomic site | <0.0001 | ||
| Lip and oral cavity | 0 (0) | 43 (47) | |
| Oropharynx | 41 (93) | 11 (12) | |
| Nasopharynx | 0 (0) | 1 (1) | |
| Hypopharynx | 0 (0) | 4 (4) | |
| Larynx | 0 (0) | 26 (29) | |
| Paranasal sinuses | 0 (0) | 5 (5) | |
| Unknown primary | 3 (6) | 1 (1) | |
| Primary treatment modality | <0.0001 | ||
| Surgery ± adjuvant radiation | 31 (70) | 87 (96) | |
| Radiation alone | 2 (5) | 3 (3) | |
| Chemoradiation | 11 (25) | 1 (1) | |
| Tumor recurrence | 0.11 | ||
| No | 35 (80) | 59 (65) | |
| Yes | 9 (20) | 32 (35) | |
| Second primary diagnosisb | |||
| No | 44 (100) | 80 (88) | 0.016 |
| Yes | 0 (0) | 11 (12) | |
22 patients did not complete the audio computer-assisted self-interview and have missing smoking and alcohol consumption data
Second primary cancers included head and neck, lung, and esophageal cancers.
Tumor specimens from 44 (33%) of 135 cases were classified as HPV16-positive by ISH (21). The characteristics of the HPV16-positive and negative cases are shown in Table 1. The HPV16-positive tumors originated from the oropharynx (n=41) or were unknown primary tumors (n=3).
Patients were followed for a median of 21 months (interquartile range, [IQR], 14–25) and provided a median of four (IQR 3–5) samples. The median number of days between samples was 132 days (IQR 90–210). The median number of samples provided by HPV16-positive patients was four (IQR 3.75–6) and by HPV16-negative patients was four (IQR 3–5).
Oral HPV infection
A total of 598 oral rinse samples were collected. One hundred thirty-three (99%) of 134 pre-treatment and 456 (98%) of 464 post-treatment samples were positive for β-globin and therefore considered evaluable. All controls for inter-specimen contamination were negative for HPV by multiplex PCR-line blot hybridization and HPV16 type-specific PCR.
The median number of oral exfoliated cells analyzed for the presence of HPV infection in oral rinse samples was 11,764 (IQR 4,520–23,634), as estimated by real-time PCR targeted to ERV-3.
Ninety-eight (16.6%) of 590 evaluable oral rinse samples were positive for HPV by line blot hybridization. Forty-six (34.1%) of the 135 cases had an oral HPV infection detected at some point during the study. An oral HPV infection was detected in 30 (22.6%) of 133 cases prior to therapy and in 37 (27.4%) of 135 cases after therapy. During the study, HPV16 was detected in 17 (12.6%), high-risk HPV types in 32 (23.7%), and low-risk types in 17 (12.6%) of the 135 case subjects.
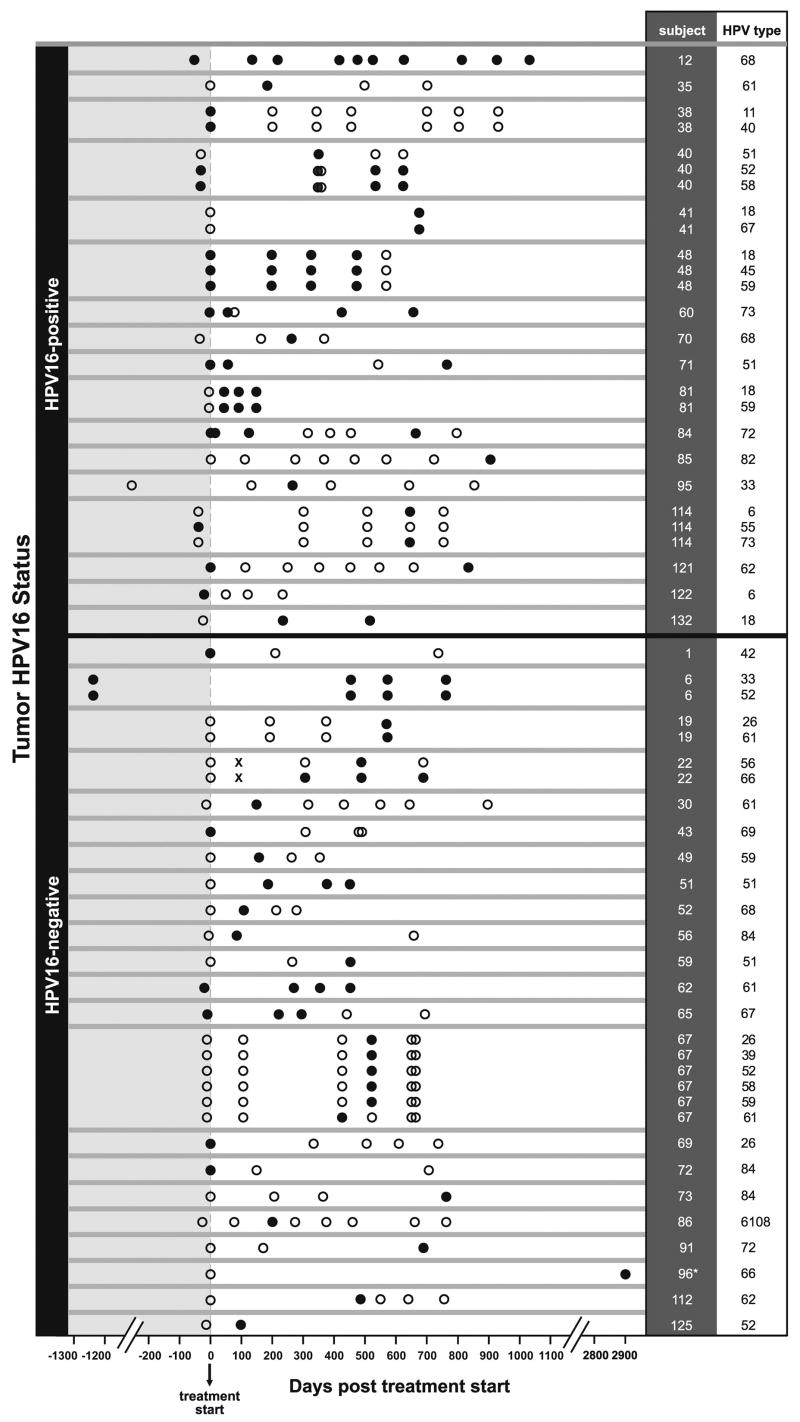
All of the oral HPV infections (other than type 16) detected during the study as well as the time frame of detection relative to the start of treatment are graphically displayed in Figure 1. Assuming repeated detection of an infection other than HPV16 represents type-specific persistence, 54 infections were detected. Nineteen of the 54 infections were detected at more than one visit, and the median number of days between the first and last visit at which an infection was detected was 560 days (IQR 370–766). For example, type-specific infection persistence was demonstrable for 10 consecutive samples collected over three years (1,086 days) in subject 12 and for four consecutive samples collected over five and a half years (2,004 days) in subject six (Figure 1). (15)
Figure One. Type-specific detection of oral HPV infection (other than HPV16) before and after therapy in patients with HPV16-positive and HPV16-negative HNSCC.
All oral HPV infections, other than type HPV16, detected during the study are shown in the figure. Data from each case (indicated by case number in the column “subject”) is delineated by horizontal gray lines. Subjects with HPV16-positive and HPV16-negative HNSCC are above and below the black horizontal line, respectively. Each row indicates a separate, type-specific infection, specified in the column labeled “HPV type.” Oral rinse samples (ORS) that were β-globin positive and therefore evaluable are represented by circles: solid black and open circles represent HPV-positive and HPV-negative ORS, respectively. β-globin negative samples are represented by an “X.” Time points for oral rinse collection relative to the start of cancer therapy are indicated on the X-axis in days. Two patients had samples that predated start of treatment by an extended period of time because of temporary loss to follow-up (subject 95) or because of a prior diagnosis of a metasynchronous head and neck cancer (subject 6). * Not shown are three HPV-negative oral rinse samples from subject 96 collected at days 2348, 2576, 2665 post treatment start.
The relationship between oral HPV infection and a diagnosis of an HPV16-positive tumor
High-risk HPV infections other than HPV16 were detected prior to therapy in 16% and 2% of cases with HPV16-positive and HPV16-negative tumors, respectively and in 27% and 13% after therapy (Table 2).
Table 2.
Detection of HPV16 and any of 36 non-HPV16 mucosal HPV types in pre and post therapy oral rinse samples by tumor HPV status (N=135)
| Tumor HPV status | ||||
|---|---|---|---|---|
| HPV16-positive N=442 | HPV16-negative N=911 | Odds Ratio (95% CI) | ||
| Oral infection | N (%) | N (%) | Unadjusted | Adjusted3 |
| HPV16 | ||||
| AT DIAGNOSIS | ||||
| Infection absent | 30 (70) | 86 (97) | 1.0 | 1.0 |
| Infection present | 13 (30) | 3 (3) | 12 (3.3, 47) | 9.6 (2.4, 39) |
| FOLLOW-UP | ||||
| Infection absent | 39 (89) | 90 (99) | 1.0 | 1.0 |
| Infection present | 5 (11) | 1 (1) | 11 (1.1, 114) | 14 (1.1, 188) |
| High risk HPV types (non-HPV16)4 | ||||
| AT DIAGNOSIS | ||||
| Infection absent | 36 (84) | 87 (98) | 1.0 | 1.0 |
| Infection present | 7 (16) | 2 (2) | 5.6 (1.4, 23) | 5.4 (1.3, 22)6 |
| FOLLOW-UP | ||||
| Infection absent | 32 (73) | 79 (87) | 1.0 | 1.0 |
| Infection present | 12 (27) | 12 (13) | 2.9 (1.1, 7.6) | 2.9 (1.4, 5.7) |
| Low risk HPV types5 | ||||
| AT DIAGNOSIS | ||||
| Infection absent | 37 (86) | 86 (97) | 1.0 | 1.0 |
| Infection present | 6 (14) | 3 (3) | 4.7 (1.7, 20) | 4.5 (1.1, 19)6 |
| FOLLOW-UP | ||||
| Infection absent | 40 (91) | 82 (90) | 1.0 | 1.0 |
| Infection present | 4 (9) | 9 (10) | 0.6 (0.2, 2.1) | 0.6 (0.2, 1.5) |
Two HPV16-negative cases had missing baseline HPV line-blot data
One HPV16-positive case had missing baseline HPV line-blot data
Adjusted for age, gender, race
High risk HPV types 18, 33, 35, 51, 52, 56, 58, 59, 67, 68, 73 detected by PCR/line blot hybridization
Low risk HPV types 6, 11, 42, 55, 61, 62, 69, 72 detected by PCR/line blot hybridization 6 Adjusted for age only due to lack of model fit with inclusion of race and gender in model
The odds of an oral HPV infection both pre and post therapy were compared among cases with HPV16-positive and negative tumors (Table 2). Cases with HPV16-positive tumors were significantly more likely than HPV16-negative cases to have an oral HPV infection detected both before (OR 8.6, 95% confidence interval, [CI], 3.5–21) and after therapy (OR 2.9, 95%CI 1.1–7.4).
Prior to therapy, oral infection by HPV16 (OR 9.6, 95%CI 2.4–39) and by other high-risk HPV types (OR 5.4, 95%CI 1.3–22) were more common in cases with HPV16-positive tumors (Table 2). The odds of low-risk HPV infection were also elevated at baseline among cases with HPV16-positive tumors (OR 4.5, 95% CI 1.1–19).
After treatment, cases with HPV16-positive tumors had an estimated 14-fold increase in odds (OR 14, 95%CI 1.1–188) of oral HPV16 infection when compared to cases with HPV16-negative tumors. The odds of infection by other high-risk HPV types were also elevated (OR 2.9, 95%CI 1.4–5.7). However, oral HPV infection by a low-risk type was not more common among patients with HPV16-positive tumors after treatment (Table 2).
A pretreatment, oral, high-risk HPV infection was significantly associated with post-treatment oral infection (OR 44, 95% CI 6.5–300). However, neither treatment modality (OR 1.3, 95% CI 0.6, 4.0) nor tumor recurrence (OR 0.9, 95% CI 0.4–2.1) were significantly associated with the presence of an oral HPV infection post-treatment. The presence of an oral HPV16 infection elevated odds of recurrence, but not significantly (OR 2.3, 95%CI 0.4–13).
Second primary tumors were diagnosed during follow-up among 11 cases, all of whom had HPV-negative tumors (Table 1). No associations were observed between the presence of an oral HPV infection at diagnosis or follow-up and a second-primary tumor (data not shown).
Variant analysis for oral rinse-tumor pairs that were HPV16-positive
To investigate the relationship between the HPV16 detected in the oral rinse samples and corresponding HPV16-positive tumors, sequencing of the E6 gene was performed. An oral HPV16 infection was detected by line blot hybridization in 16 (11%) of 133 samples at diagnosis and in a total of 6 (1%) of 456 samples collected during follow-up. The majority of oral rinse samples positive for HPV16 at baseline (13 of 16) and follow-up (5 of 6) were from individuals with HPV16-positive tumors. The median HPV16 viral load among positive samples as measured by real-time PCR was 4.6 per 1,000 cells analyzed (IQR range 0.2–19).
The E6 gene was successfully sequenced from 19 of the 22 oral rinse samples that were positive for HPV16 during baseline or follow-up (Table 3). All but one isolate (Asian) was a European variant. The most common variant was E-350T (n=6), followed by E-350G (n=4) and E-T131G (n=2). Eight of the 19 isolates were European variants with sequences unique to a single individual.
Table 3.
HPV16 variant analysis in oral rinse specimens from patients with HPV16-negative and -positive tumors and for corresponding HPV16-positive tumor samples
| Subject | Sample type | Day collected* | HPV16 Variant | Reference HPV16 E6 Sequence position, nt† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 | 131 | 178 | 256 | 307 | 315 | 350 | 478 | 506 | ||||
| HPV16-positive tumors | G | A | T | C | G | C | T | C | C | |||
| 35 | Tumor | -- | E-350T | |||||||||
| Oral rinse | 0 | E-350T | ||||||||||
| 38 | Tumor | -- | Asian | G | ||||||||
| Oral rinse | 0 | Asian | G | |||||||||
| Oral rinse | 812 | E-350G | G | |||||||||
| 41 | Tumor | -- | E-350T | del | ||||||||
| Oral rinse | 674 | E-350T | del | |||||||||
| 48 | Tumor | -- | E-350T | |||||||||
| Oral rinse | 0 | E-350T | ||||||||||
| 50 | Tumor | -- | E-350G | G | ||||||||
| Oral rinse | 480 | E-350G | G | |||||||||
| 70 | Tumor | -- | E-G256T | T | G | |||||||
| Oral rinse | -41 | E-G256T | T | G | ||||||||
| 80 | Tumor | -- | E-350T | |||||||||
| Oral rinse | 144 | E-350T | ||||||||||
| 81 | Tumor | -- | E-T131G | G | ||||||||
| Oral rinse | -6 | E-350G | G | |||||||||
| 85 | Tumor | -- | E-T315G | G | ||||||||
| Oral rinse | 0 | E-T315G | G | |||||||||
| 102 | Tumor | -- | E-G506G | G | G | |||||||
| Oral rinse | 0 | E-G506G | G | G | ||||||||
| 123 | Tumor | -- | E-T131G | G | ||||||||
| Oral rinse | -6 | E-T131G | G | |||||||||
| Oral rinse | 234 | E-350G | G | |||||||||
| 124 | Tumor | -- | E-350T | |||||||||
| Oral rinse | -6 | E-350T | ||||||||||
| 127 | Tumor | -- | E-T307A | A | ||||||||
| Oral rinse | -7 | E-T307A | A | |||||||||
| 132 | Tumor | -- | E-T131G | G | ||||||||
| Oral rinse | -24 | E-T131G | G | |||||||||
| HPV16-negative tumors | ||||||||||||
| 30 | Oral rinse | 640 | E-350T | |||||||||
| 52 | Oral rinse | 0 | E-350T | |||||||||
| 96 | Oral rinse | 0 | E-T478A | A | ||||||||
Relative to date of start of therapy, equal to day 0.
Nucleotide found at specified position in the HPV16 European reference sequence (GenBank, NC 001526). HPV16 E6 coding region is nucleotides (nt) 83–559.
Tumor DNA was purified from fresh-frozen (n=11) or paraffin (n=3) tumor samples from all patients with HPV16-positive tumors who had a positive oral rinse sample during the study (Table 3). In 10 of 11 baseline samples and in three of five follow-up samples, the sequence of the HPV16 present in the oral rinse was identical to that detected in the tumor.
Discussion
Our data indicate that oral HPV infection is significantly more common among individuals diagnosed with HPV16-positive than HPV16-negative HNSCC both before and after therapy. Infections by HPV16 as well as other high-risk types were more common in those patients with an HPV16-positive tumor, consistent with either a behavioral or biological predilection to infection.
We previously reported a strong association between oral HPV16 infection and both oropharyngeal cancer (~OR 15) and HPV16-positive HNSCC (~OR 53) in case-control studies. (4, 22) In this study, we demonstrate for the first time via sequencing that the elevated oral HPV16 infection prevalence among patients with HPV16-positive tumors at diagnosis is likely explained by tumor cells shed into the oral rinse. The sequence of all but one of the HPV16 variants was identical to that found in the corresponding tumor. We acknowledge that our laboratory methods cannot distinguish between non-infectious, intra-nuclear viral genomic DNA in tumors versus an intact, potentially infectious viral particle. Therefore, we cannot exclude the possibility of a co-extant productive viral infection that is potentially transmissible to a partner. In support of this possibility, one of 11 HPV16 variants at baseline and two of five HPV16 variants detected after therapy were distinct from that in the tumor. Although based on a small number of patients and events, our data indicate that patients may acquire oral infection by more than one HPV16 variant. Our extensive use of negative controls and repeat amplification and sequencing argues against intra-specimen contamination as the explanation for this finding.
We found no association between the presence of an oral HPV infection after therapy and tumor recurrence. This is likely because the majority of the HPV infections detected after therapy, inclusive of the HPV16 variants, were not identical to that found in the tumor. Because HPVs are extraordinarily stable DNA viruses, the presence of more than one HPV16 variant in an individual must be interpreted as distinct infections rather than acquired mutations to viral DNA. Therefore, future studies designed to evaluate whether oral HPV detection can be used to predict subsequent local tumor recurrence will need not only consider HPV type, but must specifically consider variant sequence. In patients without cancer, HPV DNA detection is usually interpreted as evidence for a productive viral infection. Thus, high-risk and low-risk infections detected in this study other than HPV16 should be considered as “infectious” and potentially transmissible to a partner.
We observed no association between the presence of an oral HPV infection and the development of a second primary tumor. As has been reported previously, second primary tumors were significantly more frequent in patients with HPV-negative tumors. (23) However, the median follow-up for patients in this study was only 21 months. We cannot exclude the possibility that second primary tumors associated with high-risk HPV infections would develop in patients with HPV infection with a longer period of observation. A diagnosis of one HPV-associated malignancy is known to elevate risk of a second, HPV-associated malignancy. (24)
Oral high-risk infections other than HPV16 were more common among patients with HPV16-positive HNSCC. We recently reported that the odds of a diagnosis of an HPV16-positive versus negative HNSCC increased with lifetime number of oral sexual partners (22). Therefore, differences in lifetime or recent sexual behavior for the HPV16-positive versus negative patient may explain the difference in infection prevalence between the two groups of patients. The time course for many of the infections we observed is unknown, as many were detected at the first and last visits. Differences in sexual behavior during the period before diagnosis among patients with HPV16-positive versus negative tumors could explain the elevated odds of low-risk infection among HPV16-positive cases at diagnosis and not after therapy. As can be seen in Figure 1, the majority of infections detected were present at diagnosis. Time to clearance of infection is longer on average for high-risk versus low-risk cervical infections (25), but it is not known whether this is the case for oral infections.
As an alternate explanation to behavioral differences, we cannot exclude the possibility of biological differences between the HPV16-positive and negative patients which may promote the long-term persistence of an oral HPV infection in the former group of patients. For instance, genetic variation in HLA Class I and II genes (26) and chemokines (27) may influence the cell-mediated clearance of infection and therefore risk of SCC. We also cannot exclude the possibility that reactivation of latent infection may occur as a consequence of radiation-induced oral mucositis and repair. However, almost all of the HPV16-positive and negative patients were treated with radiotherapy and the majority of samples were collected more than three months post-initiation of therapy. Therefore, this is unlikely to account for the differences observed between the HPV16-positive and negative patients.
We acknowledge the limitations of our study, as this study was not specifically designed for this analysis. The number of samples collected varied considerably from person to person and may have introduced systematic bias that could affect the data in unpredictable ways. However, the median number of samples collected from patients with HPV16-positive and negative tumors was the same and statistical methods were used to account for correlated observations within individuals. For our description of oral HPV infection over time shown in Figure 1, we assumed that repeated detection of an identical HPV type other than HPV16 was indicative of a persistent infection. Given our data that more than one HPV16 variant could be detected over time in the same individual, this assumption may be incorrect for other types. Additionally, tumors were classified as HPV-positive based on the results of HPV16 in situ hybridization alone. As approximately 5% of HPV-positive tumors are positive for high-risk types other than HPV16, we may have misclassified some HPV-positive tumors as HPV-negative. However, given this study included only 12 cases of HPV16-negative oropharynx or unknown primary tumors, the effect of any misclassification on our data would likely be minimal. Furthermore, odds of infection among HPV16-positive HNSCC cases were compared to HPV-negative HNSCC cases, after consideration of age and gender, rather than age and gender matched controls. However, the point estimates in this study are likely a reasonable approximation for what would be observed in comparison to controls, given the odds of HPV infection among HPV-negative cases have not been significantly elevated when compared to controls in our prior work. (22)
Despite these limitations, our data indicate that high-risk oral HPV infections are more common both before and after therapy among patients with HPV16-positive HNSCC as compared to patients with HPV16-negative HNSCC, consistent with their having either a behavioral and/or biological disposition to infection. This is also the first study to demonstrate type-specific persistence of oral HPV infection beyond six months in human subjects via collection of consecutive oral rinse samples, albeit in a small number of individuals. Infection persistence was observed in some subjects for several years. The data indicate that it is possible to study the natural history of oral HPV infection and factors that affect the incidence and persistence of infection with our methods. These methods can therefore be applied to larger studies specifically designed to study the natural history of oral HPV infection in this patient population and associations with disease outcomes, as well as possible transmission to a partner. Such studies are clearly needed, as medical professionals tackle a barrage of questions concerning the psycho-social implications of a diagnosis of HPV16-positive HNSCC.
Acknowledgments
Maura L Gillison (NIDCR DE016631, Oral Cancer Foundation) Wayne Koch (NIH 1 P50 CA96784-01) David Symer (This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U. S. Government.)
Footnotes
Statement of Clinical Relevance
Oral HPV infection is now recognized as an important cause of oropharyngeal cancers. Detection of oral HPV infection may therefore have future clinical utility for identifying individuals at risk for oropharyngeal cancer or in early detection of local recurrence in patients with a diagnosis of HPV-positive oropharyngeal cancer. Such potential clinical utility depends upon methods for detection and identification of oral HPV infection over time. To that end, in this manuscript it is demonstrated that: (1) serial oral rinse samples permit detection of persistent, type-specific, oral HPV infection for as long as five years; (2) the HPV16 variant sequence in oral rinse samples collected before and after cancer therapy is usually, although not always, identical to that in the tumor; (3) oral, high-risk HPV infections other than HPV16 are common before and after therapy among patients with HPV16-positive oropharyngeal cancer; and (4) second primary cancers of the aerodigestive tract are less frequent among patients with HPV16-positive versus negative cancers. Therefore, type-specific detection of oral HPV infection utilizing the methods in this manuscript can facilitate the study of the clinical consequences of infection, including associations with disease outcomes and second primary cancers.
References
- 1.Hansson BG, Rosenquist K, Antonsson A, et al. Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1337–44. doi: 10.1080/00016480510043945. [DOI] [PubMed] [Google Scholar]
- 2.Pintos J, Black MJ, Sadeghi N, et al. Human papillomavirus infection and oral cancer: a case-control study in Montreal, Canada. Oral Oncol. 2008;44:242–50. doi: 10.1016/j.oraloncology.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Smith EM, Ritchie JM, Summersgill KF, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–55. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 6.Coutlee F, Trottier AM, Ghattas G, et al. Risk factors for oral human papillomavirus in adults infected and not infected with human immunodeficiency virus. Sex Transm Dis. 1997;24:23–31. doi: 10.1097/00007435-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189:686–98. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 8.Rintala M, Grenman S, Puranen M, Syrjanen S. Natural history of oral papillomavirus infections in spouses: a prospective Finnish HPV Family Study. J Clin Virol. 2006;35:89–94. doi: 10.1016/j.jcv.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Hemminki K, Dong C. Cancer in husbands of cervical cancer patients. Epidemiology. 2000;11:347–9. doi: 10.1097/00001648-200005000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Hemminki K, Dong C, Frisch M. Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev. 2000;9:433–7. doi: 10.1097/00008469-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Rose Ragin CC, Taioli E. Second primary head and neck tumor risk in patients with cervical cancer--SEER data analysis. Head Neck. 2008;30:58–66. doi: 10.1002/hed.20663. [DOI] [PubMed] [Google Scholar]
- 12.Spitz MR, Sider JG, Schantz SP, Newell GR. Association between malignancies of the upper aerodigestive tract and uterine cervix. Head Neck. 1992;14:347–51. doi: 10.1002/hed.2880140502. [DOI] [PubMed] [Google Scholar]
- 13.Haddad R, Crum C, Chen Z, et al. HPV16 transmission between a couple with HPV-related head and neck cancer. Oral Oncol. 2007 doi: 10.1016/j.oraloncology.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–75. [PubMed] [Google Scholar]
- 15.D’Souza G, Sugar E, Ruby W, Gravitt P, Gillison M. Analysis of the effect of DNA purification on detection of human papillomavirus in oral rinse samples by PCR. J Clin Microbiol. 2005;43:5526–35. doi: 10.1128/JCM.43.11.5526-5535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutlee F, Gravitt P, Kornegay J, et al. Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol. 2002;40:902–7. doi: 10.1128/JCM.40.3.902-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved mplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravitt PE, Peyton C, Wheeler C, Apple R, Higuchi R, Shah KV. Reproducibility of HPV 16 and HPV 18 viral load quantitation using TaqMan real-time PCR assays. J Virol Methods. 2003;112:23–33. doi: 10.1016/s0166-0934(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T, Manos MM, Peto J, et al. Human papillomavirus type 16 sequence variation in cervical cancers: a worldwide perspective. J Virol. 1997;71:2463–72. doi: 10.1128/jvi.71.3.2463-2472.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada T, Wheeler CM, Halpern AL, Stewart AC, Hildesheim A, Jenison SA. Human papillomavirus type 16 variant lineages in United States populations characterized by nucleotide sequence analysis of the E6, L2, and L1 coding segments. J Virol. 1995;69:7743–53. doi: 10.1128/jvi.69.12.7743-7753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CC, Qiu JT, Kashima ML, Kurman RJ, Wu TC. Generation of type-specific probes for the detection of single-copy human papillomavirus by a novel in situ hybridization method. Mod Pathol. 1998;11:971–7. [PubMed] [Google Scholar]
- 22.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 23.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–6. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 24.Frisch M, Biggar RJ. Aetiological parallel between tonsillar and anogenital squamous-cell carcinomas. Lancet. 1999;354:1442–3. doi: 10.1016/S0140-6736(99)92824-6. [DOI] [PubMed] [Google Scholar]
- 25.Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–92. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madeleine MM, Brumback B, Cushing-Haugen KL, et al. Human leukocyte antigen class II and cervical cancer risk: a population-based study. J Infect Dis. 2002;186:1565–74. doi: 10.1086/345285. [DOI] [PubMed] [Google Scholar]
- 27.Ivansson EL, Gustavsson IM, Magnusson JJ, et al. Variants of chemokine receptor 2 and interleukin 4 receptor, but not interleukin 10 or Fas ligand, increase risk of cervical cancer. Int J Cancer. 2007;121:2451–7. doi: 10.1002/ijc.22989. [DOI] [PubMed] [Google Scholar]