Abstract
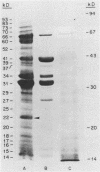
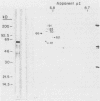
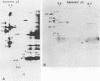
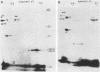
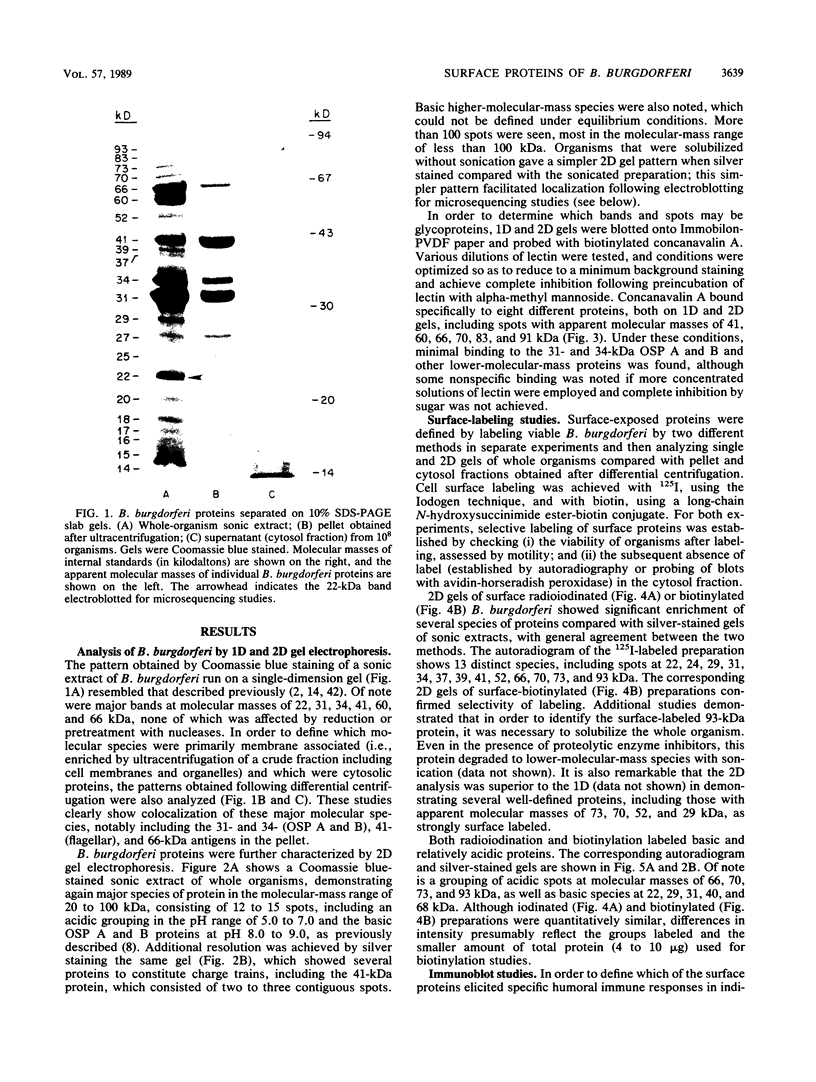
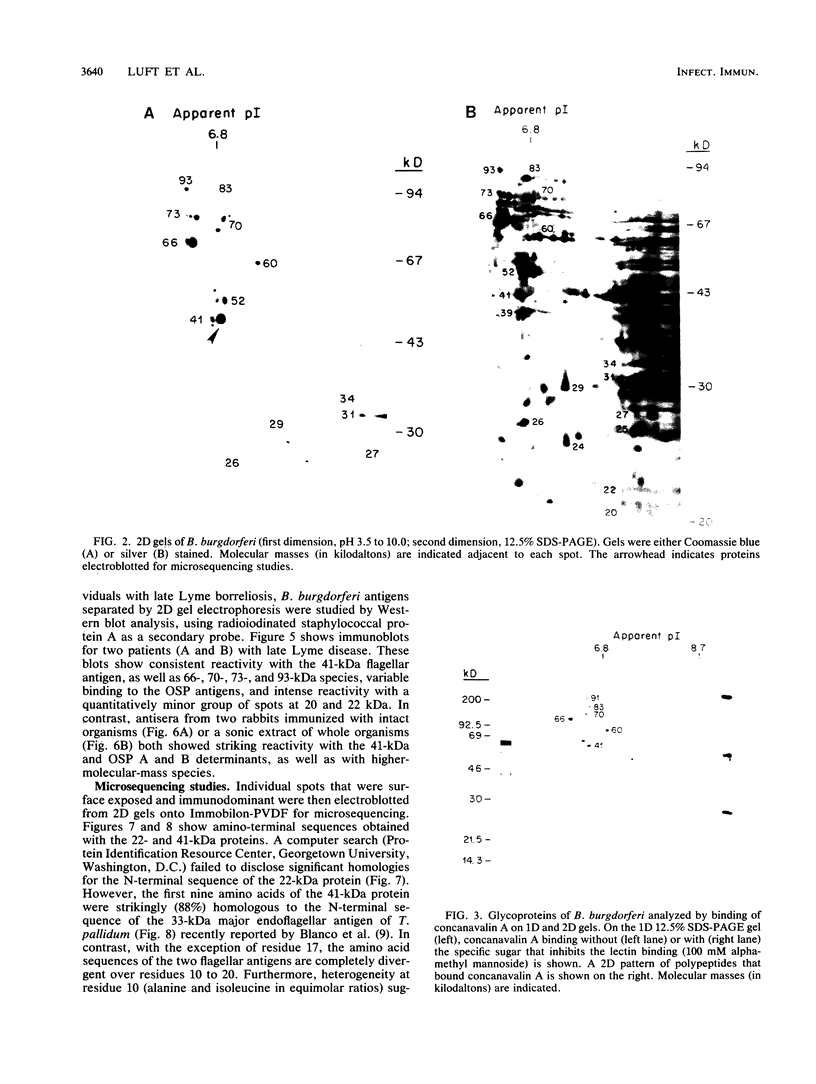
The immunodominant proteins and glycoproteins of Borrelia burgdorferi were analyzed by one-dimensional (1D) and 2D gel electrophoresis. More than 100 polypeptide species could be detected on silver-stained 2D gels. Separation of sonic extracts of the organism by differential centrifugation (100,000 X g) revealed several of the major proteins to reside predominantly within the pellet fraction. The antigenicity of the individual polypeptides was determined by Western (immuno-) blot analysis with sera from humans with chronic Lyme disease and from rabbits immunized with B. burgdorferi. Surface proteins of viable B. burgdorferi labeled with 125I or long-arm hydroxysuccinimide biotin were identified by gel analyses. Thirteen major surface proteins were apparent, including the highly immunogenic 41-kilodalton (kDa) endoflagellar antigen. Two of these proteins, with molecular masses of 22 and 41 kDa, were further characterized by electroblotting and microsequencing their amino termini. Significant (35%) homology between the first 20 amino acids of the 22-kDa protein and the deduced amino acid sequence of the 31-kDa (outer surface protein A) protein of B. burgdorferi may indicate that these proteins are processed similarly or are part of a gene family expressed at the surface of the organism. In addition, highly significant (88%) homology was found between the first nine amino acids of the 41-kDa protein of B. burgdorferi and the 33-kDa endoflagellar protein of Treponema pallidum, after which the sequences diverge. This observation provides in part a structural basis for the observed cross-reactivity between the two organisms and suggests alternative approaches to the development of specific immunodiagnostics.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. The genes encoding major surface proteins of Borrelia burgdorferi are located on a plasmid. Ann N Y Acad Sci. 1988;539:144–153. doi: 10.1111/j.1749-6632.1988.tb31847.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Golightly M. G. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of the Lyme disease spirochete. J Immunol. 1988 Jan 1;140(1):265–272. [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Blanco D. R., Champion C. I., Miller J. N., Lovett M. A. Antigenic and structural characterization of Treponema pallidum (Nichols strain) endoflagella. Infect Immun. 1988 Jan;56(1):168–175. doi: 10.1128/iai.56.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco D. R., Radolf J. D., Lovett M. A., Miller J. N. Correlation of treponemicidal activity in normal human serum with the presence of IgG antibody directed against polypeptides of Treponema phagedenis biotype Reiter and Treponema pallidum, Nichols strain. J Immunol. 1986 Sep 15;137(6):2031–2036. [PubMed] [Google Scholar]
- Blanco D. R., Radolf J. D., Lovett M. A., Miller J. N. The antigenic interrelationship between the endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum Nichols strain. I. Treponemicidal activity of cross-reactive endoflagellar antibodies against T. pallidum. J Immunol. 1986 Nov 1;137(9):2973–2979. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Chang J. Y., Shaper J. H., Glazer A. N. Amino acid sequence of flagellin of Bacillus subtilis 168. III. Tryptic peptides, N-bromosuccinimide peptides, and the complete amino acid sequence. J Biol Chem. 1976 Feb 10;251(3):705–711. [PubMed] [Google Scholar]
- Gavine F. S., Pryde J. G., Deane D. L., Apps D. K. Glycoproteins of the chromaffin granule membrane: separation by two-dimensional electrophoresis and identification by lectin binding. J Neurochem. 1984 Nov;43(5):1243–1252. doi: 10.1111/j.1471-4159.1984.tb05379.x. [DOI] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Use of lectins for detection of electrophoretically separated glycoproteins transferred onto nitrocellulose sheets. Anal Biochem. 1981 Jul 15;115(1):219–224. doi: 10.1016/0003-2697(81)90549-2. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin M., Rottem S., Razin S. The outer membrane of Proteus mirabilis. I. Isolation and characterization of the outer and cytoplasmic membrane fractions. Biochim Biophys Acta. 1975 Feb 14;375(3):381–394. doi: 10.1016/0005-2736(75)90354-5. [DOI] [PubMed] [Google Scholar]
- Heitzmann H., Richards F. M. Use of the avidin-biotin complex for specific staining of biological membranes in electron microscopy. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3537–3541. doi: 10.1073/pnas.71.9.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Lactoperoxidase and Iodo-Gen-catalyzed iodination labels inner and outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Jul;155(1):443–446. doi: 10.1128/jb.155.1.443-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Norris S. J., Charon N. W., Cook R. G., Fuentes M. D., Limberger R. J. Antigenic relatedness and N-terminal sequence homology define two classes of periplasmic flagellar proteins of Treponema pallidum subsp. pallidum and Treponema phagedenis. J Bacteriol. 1988 Sep;170(9):4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Sell S. Antigenic complexity of Treponema pallidum: antigenicity and surface localization of major polypeptides. J Immunol. 1984 Nov;133(5):2686–2692. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Electrophoretic patterns of membrane proteins of Mycoplasma. J Bacteriol. 1967 Aug;94(2):359–364. doi: 10.1128/jb.94.2.359-364.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Nice E. C. In situ cyanogen bromide cleavage of N-terminally blocked proteins in a gas-phase sequencer. Biochem Int. 1984 Jun;8(6):787–791. [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Hodinka R. L., Wyrick P. B., Bassford P. J., Jr Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987 Sep;55(9):2255–2261. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. H., Williams R. P. Use of iodo-gen and iodine-125 to label the outer membrane proteins of whole cells of Neisseria gonorrhoeae. Anal Biochem. 1982 Mar 1;120(2):254–258. doi: 10.1016/0003-2697(82)90344-x. [DOI] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. N., Joys T. M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985 Dec 20;186(4):791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]