Abstract
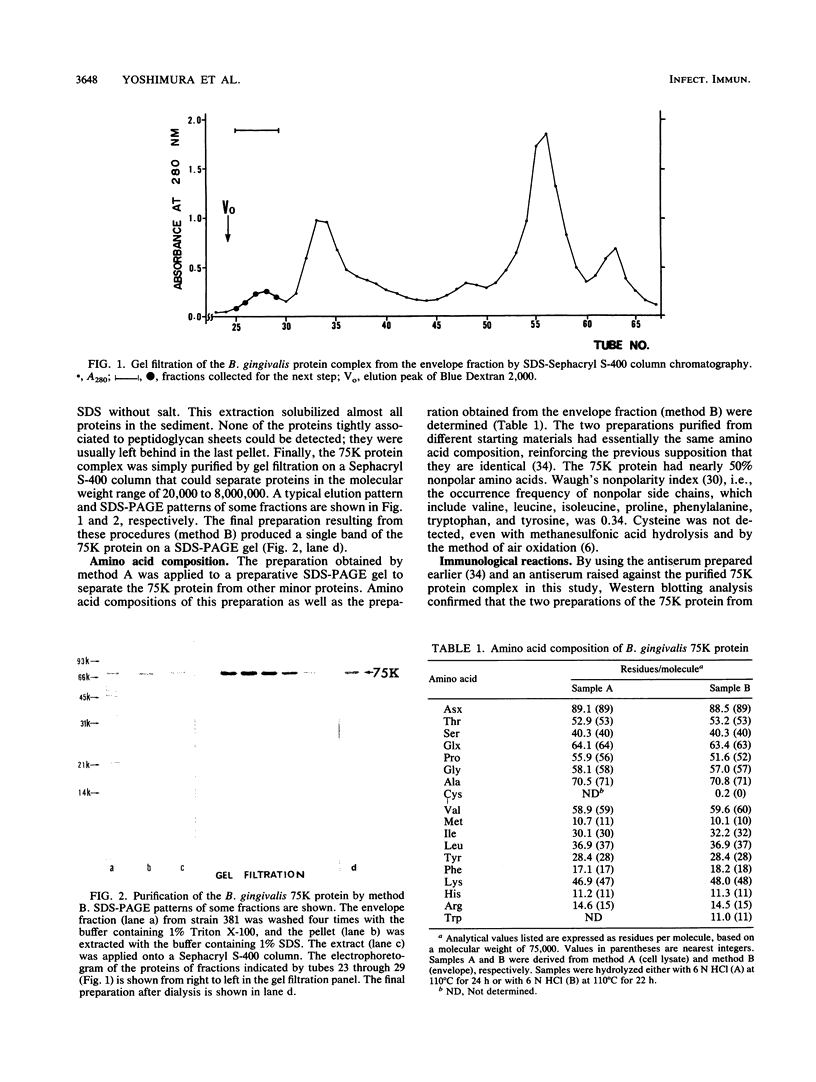
A 75-kilodalton major protein (75K protein) was purified to homogeneity from the cell lysate fraction and the envelope of Bacteroides gingivalis 381. The 75K protein was originally present in the outer membrane or the outermost part of this organism as a large, stable complex with an apparent molecular weight of about 2,000,000. Heating at 80 degrees C and at higher temperatures in the presence of sodium dodecyl sulfate was needed to completely dissociate it to monomers. Amino acid analysis revealed that the 75K protein had about 50% nonpolar amino acids. Various strains of B. gingivalis but not other bacteria, including oral Bacteroides species tested, contained serologically related 75K proteins when tested in Western blotting (immunoblotting) analysis. The abundance and localization of the 75K protein in this organism suggest that it has the potential to participate in the host-parasite interaction in infection. The 75K protein was, indeed, strongly recognized in patients with adult periodontal diseases. Immunoblotting with sera from patients and with rabbit antisera generated by intravenous inoculations of whole B. gingivalis cells revealed that the 75K protein was an immunodominant antigen on the surface of B. gingivalis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deal C. D., Kaplan S. Solubilization, isolation, and immunochemical characterization of the major outer membrane protein from Rhodopseudomonas sphaeroides. J Biol Chem. 1983 May 25;258(10):6524–6529. [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Nishihara T., Koga T., Hamada S. Serological properties and immunobiological activities of lipopolysaccharides from black-pigmented and related oral Bacteroides species. J Gen Microbiol. 1988 Nov;134(11):2867–2876. doi: 10.1099/00221287-134-11-2867. [DOI] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H., STEIN W. H., MOORE S. The amino acid composition of ribonuclease. J Biol Chem. 1954 Dec;211(2):941–950. [PubMed] [Google Scholar]
- Hitchcock P. J., Leive L., Mäkelä P. H., Rietschel E. T., Strittmatter W., Morrison D. C. Lipopolysaccharide nomenclature--past, present, and future. J Bacteriol. 1986 Jun;166(3):699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Koga T., Nishihara T., Fujiwara T., Nisizawa T., Okahashi N., Noguchi T., Hamada S. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985 Mar;47(3):638–647. doi: 10.1128/iai.47.3.638-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Coleman S. E. Immunochemical differences between oral and nonoral strains of Bacteroides asaccharolyticus. Infect Immun. 1980 Feb;27(2):589–596. doi: 10.1128/iai.27.2.589-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansheim B. J., Kasper D. L. Purification and immunochemical characterization of the outer membrane complex of Bacteroides melaninogenicus subspecies asaccharolyticus. J Infect Dis. 1977 May;135(5):787–799. doi: 10.1093/infdis/135.5.787. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Onderdonk A. B., Kasper D. L. Immunochemical and biologic studies of the lipopolysaccharide of Bacteroides melaninogenicus subspecies asaccharolyticus. J Immunol. 1978 Jan;120(1):72–78. [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yoshimura F., Takahashi K., Tani H., Suzuki T. Detection of fimbriae and fimbrial antigens on the oral anaerobe Bacteroides gingivalis by negative staining and serological methods. J Gen Microbiol. 1988 Oct;134(10):2713–2720. doi: 10.1099/00221287-134-10-2713. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAUGH D. F. Protein-protein interactions. Adv Protein Chem. 1954;9:325–437. doi: 10.1016/s0065-3233(08)60210-7. [DOI] [PubMed] [Google Scholar]
- Williams G. D., Holt S. C. Characteristics of the outer membrane of selected oral Bacteroides species. Can J Microbiol. 1985 Mar;31(3):238–250. doi: 10.1139/m85-046. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Sugano T., Kawanami M., Kato H., Suzuki T. Detection of specific antibodies against fimbriae and membrane proteins from the oral anaerobe Bacteroides gingivalis in patients with periodontal diseases. Microbiol Immunol. 1987;31(9):935–941. doi: 10.1111/j.1348-0421.1987.tb03154.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takasawa T., Yoneyama M., Yamaguchi T., Shiokawa H., Suzuki T. Fimbriae from the oral anaerobe Bacteroides gingivalis: physical, chemical, and immunological properties. J Bacteriol. 1985 Aug;163(2):730–734. doi: 10.1128/jb.163.2.730-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]
- van der Ley P., de Graaff P., Tommassen J. Shielding of Escherichia coli outer membrane proteins as receptors for bacteriophages and colicins by O-antigenic chains of lipopolysaccharide. J Bacteriol. 1986 Oct;168(1):449–451. doi: 10.1128/jb.168.1.449-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]