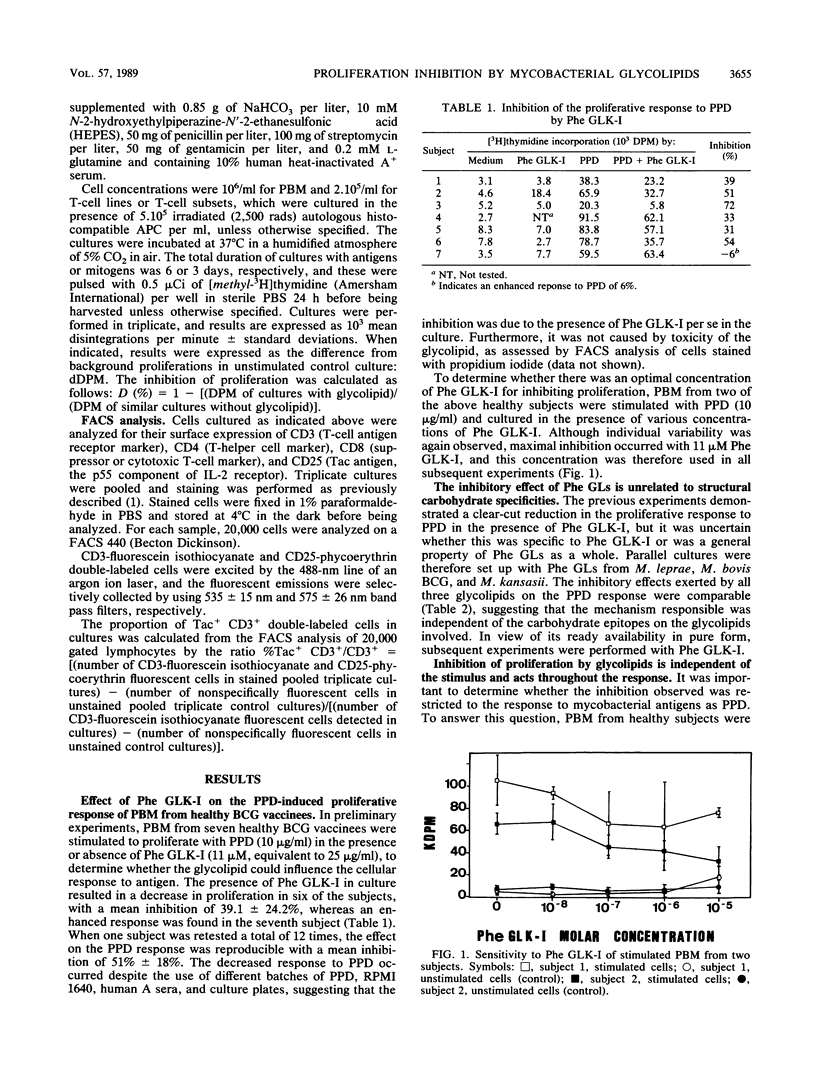
Abstract
The effect of mycobacterial phenolic glycolipids from Mycobacterium leprae, M. bovis BCG, and M. kansasii on in vitro proliferative responses by human blood mononuclear cells from healthy BCG vaccinees was investigated. All three phenolic glycolipids inhibited proliferation in a concentration-dependent manner. Inhibition was independent of the stimulus used and involved neither antigen-presenting cells nor antigen-specific CD8+ suppressor T cells. It was concluded that the phenomenon may be a general property of mycobacterial phenolic glycolipids, perhaps analogous to the growth-modulating properties of gangliosides. Despite the lack of specificity of inhibition in vitro, de facto specificity may occur in vivo by virtue of the localization of glycolipid in the leprosy lesions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E., Wotherspoon J., Hellqvist L., Basten A. High dose suppression of human anti-influenza A virus responses using T cell clones. Immunol Cell Biol. 1987 Feb;65(Pt 1):25–33. doi: 10.1038/icb.1987.3. [DOI] [PubMed] [Google Scholar]
- Baumgart K., Britton W., Basten A., Bagshawe A. Use of phenolic glycolipid 1 for serodiagnosis of leprosy in a high prevalence village in Papua New Guinea. Trans R Soc Trop Med Hyg. 1987;81(6):1030–1032. doi: 10.1016/0035-9203(87)90388-9. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Lowe C., Payne S. N., Draper P. Phenolic glycolipid 1 of Mycobacterium leprae causes nonspecific inflammation but has no effect on cell-mediated responses in mice. Infect Immun. 1984 Dec;46(3):802–808. doi: 10.1128/iai.46.3.802-808.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Brownback P. E., Barrow W. W. Modified lymphocyte response to mitogens after intraperitoneal injection of glycopeptidolipid antigens from Mycobacterium avium complex. Infect Immun. 1988 May;56(5):1044–1050. doi: 10.1128/iai.56.5.1044-1050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Fujiwara T., Brennan P., McNeil M., Turco S. J., Sibille J. C., Snapper M., Aisen P., Bloom B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. N., Hunter S. W., Gelber R. H., Rea T. H., Brennan P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J Infect Dis. 1986 Mar;153(3):560–569. doi: 10.1093/infdis/153.3.560. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutz D. J., Chen T. S., Lu W. H. The continuous bacteremia of lepromatous leprosy. N Engl J Med. 1972 Jul 27;287(4):159–164. doi: 10.1056/NEJM197207272870402. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Fournie J. J., Riviere M., Puzo G. Absolute configuration of the unique 2,6-dideoxy-4-O-methyl-arabino-hexopyranose of the major phenolic glycolipid antigen from Mycobacterium kansasii. Eur J Biochem. 1987 Oct 1;168(1):181–183. doi: 10.1111/j.1432-1033.1987.tb13402.x. [DOI] [PubMed] [Google Scholar]
- Fournié J. J., Rivière M., Puzo G. Structural elucidation of the major phenolic glycolipid from Mycobacterium kansasii. I. Evidence for tetrasaccharide structure of the oligosaccharide moiety. J Biol Chem. 1987 Mar 5;262(7):3174–3179. [PubMed] [Google Scholar]
- Hooper L. C., Johnson M. M., Khera V. R., Barrow W. W. Macrophage uptake and retention of radiolabeled glycopeptidolipid antigens associated with the superficial L1 layer of Mycobacterium intracellulare serovar 20. Infect Immun. 1986 Oct;54(1):133–141. doi: 10.1128/iai.54.1.133-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldany R. R., Maasho K., Ohman R., Reitz-Vick D., Britton S., Lefford M. J. Methods for the detection of a specific Mycobacterium leprae antigen in the urine of leprosy patients. Scand J Immunol. 1987 Jan;25(1):37–43. doi: 10.1111/j.1365-3083.1987.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Koster F. T., Teuscher C., Matzner P., Umland E., Yanagihara D., Brennan P. J., Tung K. S. Strain variations in the murine cellular immune response to the phenolic glycolipid I antigen of Mycobacterium leprae. Infect Immun. 1986 Feb;51(2):495–500. doi: 10.1128/iai.51.2.495-500.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine R. A., Hakomori S. Incorporation of exogenous glycosphingolipids in plasma membranes of cultured hamster cells and concurrent change of growth behavior. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1039–1045. doi: 10.1016/0006-291x(73)90798-5. [DOI] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Rothman W., Reinherz E., Schlossman S. F., Bloom B. R. Delineation of a human T cell subset responsible for lepromin-induced suppression in leprosy patients. J Immunol. 1980 Sep;125(3):1183–1188. [PubMed] [Google Scholar]
- Moreno C., Mehlert A., Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988 Nov;74(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Neill M. A., Klebanoff S. J. The effect of phenolic glycolipid-1 from Mycobacterium leprae on the antimicrobial activity of human macrophages. J Exp Med. 1988 Jan 1;167(1):30–42. doi: 10.1084/jem.167.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H. K., Mishra R. S., Nath I. Phenolic glycolipid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin A responses unrelated to leprosy type. J Exp Med. 1987 Jan 1;165(1):239–244. doi: 10.1084/jem.165.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Vachula M., Holzer T. J., Andersen B. R. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae. J Immunol. 1989 Mar 1;142(5):1696–1701. [PubMed] [Google Scholar]
- Venkatesan K., Singh H., Bharadwaj V. P., Ramu G. Isolation, purification and quantification of phenolic glycolipid-1 from human leprosy skin tissues. Trans R Soc Trop Med Hyg. 1988;82(2):321–323. doi: 10.1016/0035-9203(88)90461-0. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]



