Abstract
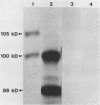
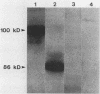
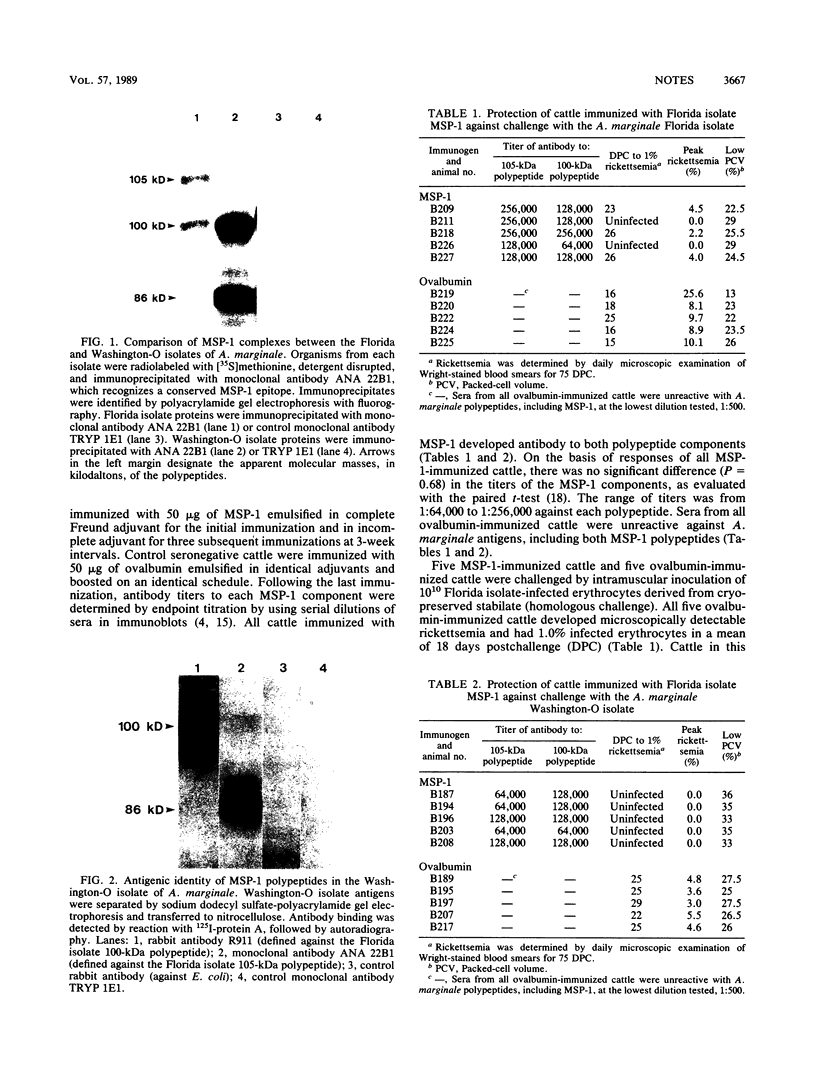
The Anaplasma marginale surface protein complex MSP-1 of the Florida isolate is composed of a 105-kilodalton (kDa) polypeptide, which bears a neutralization-sensitive epitope, and a 100-kDa polypeptide. Antigenically similar polypeptides in the Okanogan, Wash. (Washington-O), isolate MSP-1 are 86 and 100 kDa, respectively. Immunization of cattle with Florida isolate MSP-1 induced antibody titers to both MSP-1 polypeptides and protected cattle against homologous and heterologous challenge.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbet A. F., Anderson L. W., Palmer G. H., McGuire T. C. Comparison of proteins synthesized by two different isolates of Anaplasma marginale. Infect Immun. 1983 Jun;40(3):1068–1074. doi: 10.1128/iai.40.3.1068-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck L., Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984 Aug;133(2):969–974. [PubMed] [Google Scholar]
- Hanson B. A. Effect of immune serum on infectivity of Rickettsia tsutsugamushi. Infect Immun. 1983 Oct;42(1):341–349. doi: 10.1128/iai.42.1.341-349.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. X. Morphologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:676–687. [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. XI. Immunoserologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:688–696. [PubMed] [Google Scholar]
- Kuttler K. L., Zaugg J. L., Johnson L. W. Serologic and clinical responses of premunized, vaccinated, and previously infected cattle to challenge exposure by two different Anaplasma marginale isolates. Am J Vet Res. 1984 Nov;45(11):2223–2226. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F., McGuire T. C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988 Jun;56(6):1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Musoke A. J., Katende J. M., Rurangirwa F., Shkap V., Pipano E., Davis W. C., McGuire T. C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988 Feb;18(1):33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Palmer G. H., Oberle S. M., Barbet A. F., Goff W. L., Davis W. C., McGuire T. C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988 Jun;56(6):1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Waghela S. D., Barbet A. F., Davis W. C., McGuire T. C. Characterization of a neutralization-sensitive epitope on the Am 105 surface protein of Anaplasma marginale. Int J Parasitol. 1987 Oct;17(7):1279–1285. doi: 10.1016/0020-7519(87)90093-2. [DOI] [PubMed] [Google Scholar]
- Ristic M., Carson C. A. Methods of immunoprophylaxis against bovine anaplasmosis with emphasis on use of the attenuated Anaplasma marginale vaccine. Adv Exp Med Biol. 1977;93:151–188. doi: 10.1007/978-1-4615-8855-9_10. [DOI] [PubMed] [Google Scholar]
- Tamura A., Ohashi N., Urakami H., Takahashi K., Oyanagi M. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun. 1985 Jun;48(3):671–675. doi: 10.1128/iai.48.3.671-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino O., Corrier D. E., Terry M. K., Carson C. A., Lee A. J., Kuttler K. L., Ristic M., Treviño G. S. Comparison of three methods of immunization against bovine anaplasmosis: evaluation of protection afforded against field challenge exposure. Am J Vet Res. 1980 Jul;41(7):1066–1068. [PubMed] [Google Scholar]