Abstract
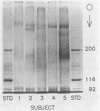
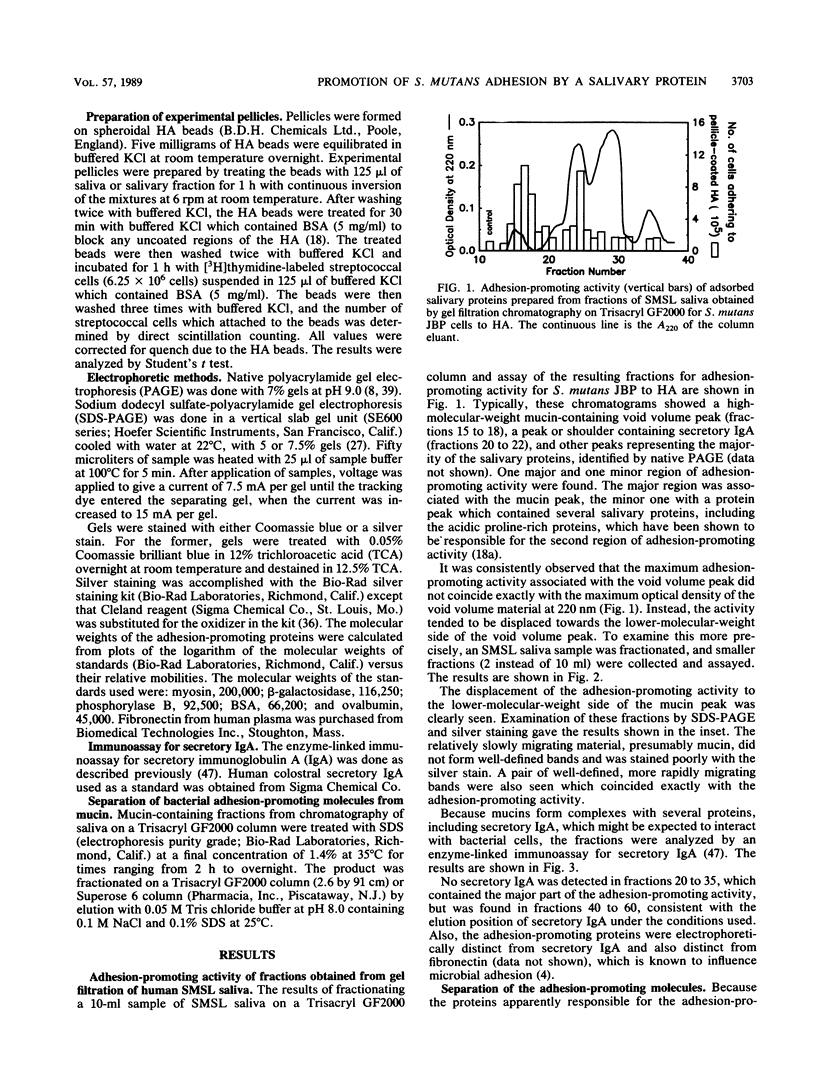
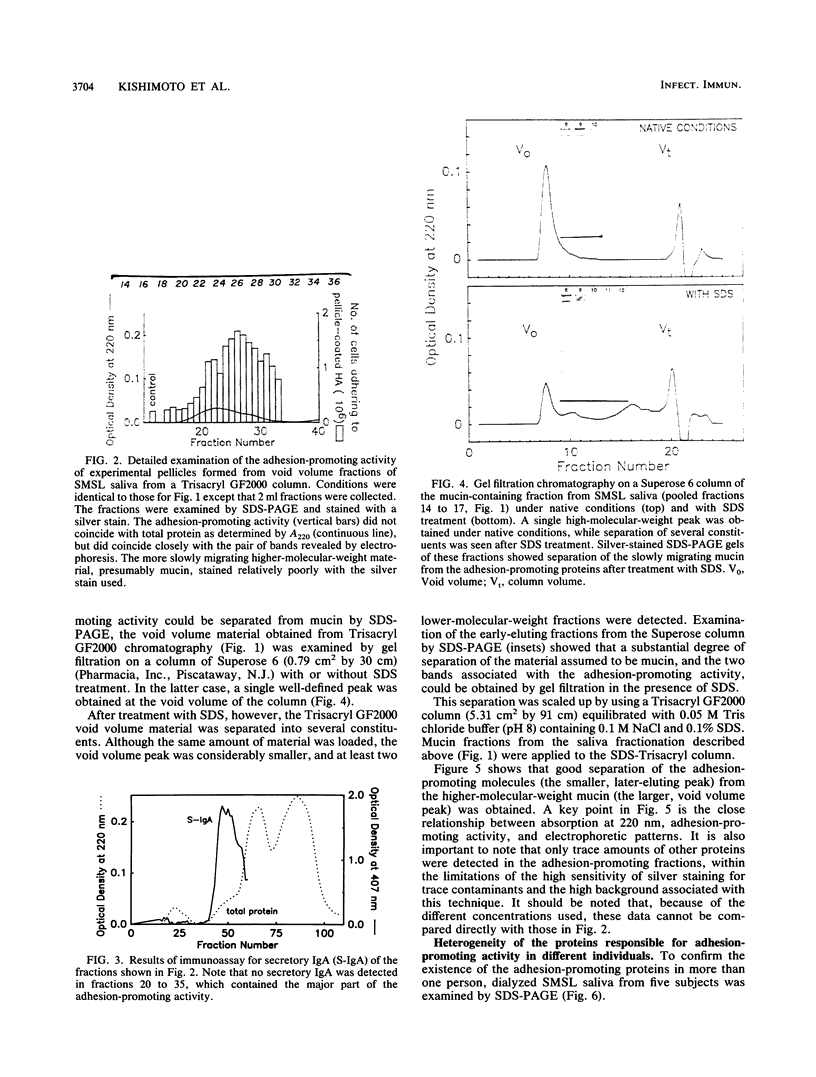
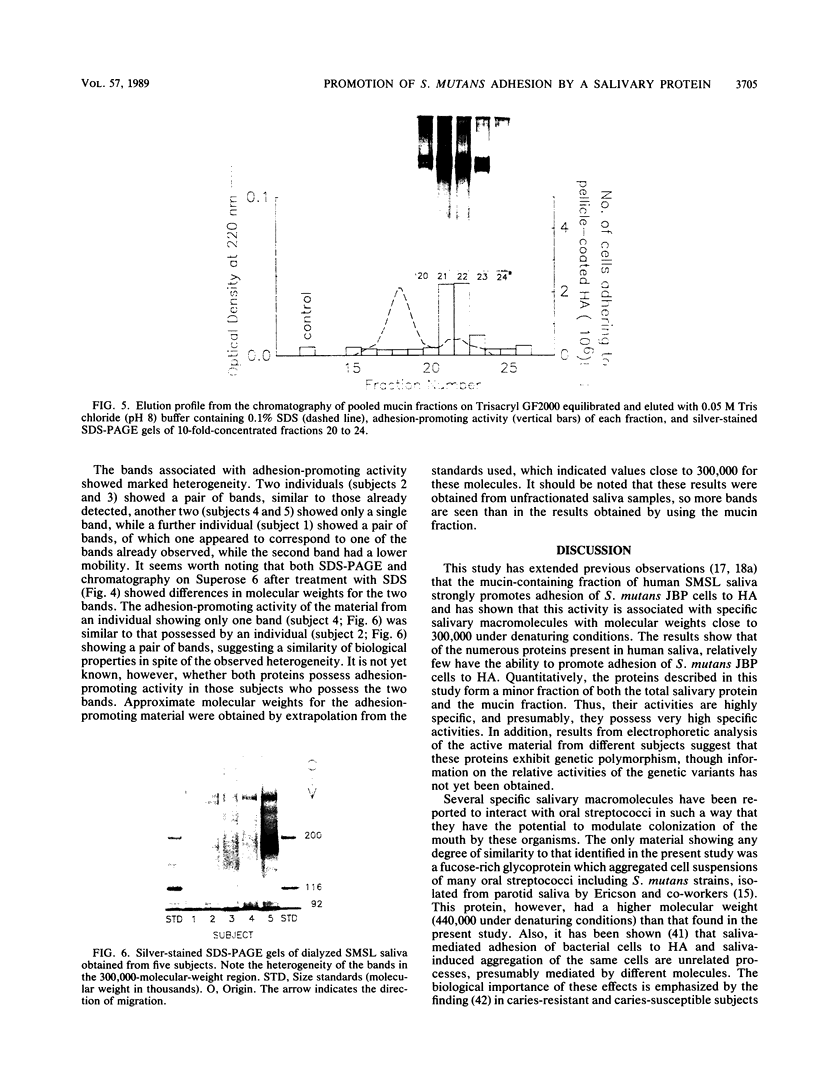
The aim of this study was to investigate the nature of one of the factors in human submandibular-sublingual (SMSL) saliva which promotes the adhesion of Streptococcus mutans serotype c strains to hydroxyapatite (HA) surfaces. Gel filtration chromatography of SMSL saliva on Trisacryl GF2000 gave a void volume peak which contained the major fraction of adhesion-promoting activity for S. mutans JBP to HA. Maximum adhesion-promoting activity, however, eluted slightly later than the maximum 220-nm absorbance of the void volume peak. Gel filtration of the void volume material after treatment with sodium dodecyl sulfate (SDS) gave an early-eluting larger peak followed by a smaller peak with which the adhesion-promoting activity was associated. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) showed the presence of relatively slowly migrating material associated with the larger inactive peak, presumably mucin, and a faster-migrating band(s) associated with the smaller active peak. SDS-PAGE indicated molecular weights in the range of 300,000 to 350,000 by extrapolation from size standards. Comparison of SMSL from five individuals showed the presence of single bands or double bands associated with adhesion-promoting activity, indicating genetic polymorphism. The active material did not resemble either secretory immunoglobulin A, based on SDS-PAGE and immunoassay, or fibronectin, based on SDS-PAGE, and also differed in molecular weight from salivary mucins and salivary constituents previously reported to promote aggregation of certain oral bacteria, but a relationship to these materials cannot be excluded. This adhesion-promoting material may play a significant role in the initial colonization of tooth surfaces by S. mutans strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M. R., Burns C. A., Arnold R. R. Comparison fo agglutinin titers for Streptococcus mutans in tears, saliva, and serum. Infect Immun. 1982 Jan;35(1):202–204. doi: 10.1128/iai.35.1.202-204.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Hasty D. L., Simpson W. A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986 Feb;51(2):405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Simpson W. A. Inhibition of the interaction of Streptococcus sanguis with hexadecane droplets by 55- and 60-kilodalton hydrophobic proteins of human saliva. Infect Immun. 1986 Aug;53(2):278–284. doi: 10.1128/iai.53.2.278-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Dabbous M. K. Interaction of salivary fibronectin with oral streptococci. J Dent Res. 1986 Aug;65(8):1094–1100. doi: 10.1177/00220345860650081001. [DOI] [PubMed] [Google Scholar]
- Babu J. P., Simpson W. A., Courtney H. S., Beachey E. H. Interaction of human plasma fibronectin with cariogenic and non-cariogenic oral streptococci. Infect Immun. 1983 Jul;41(1):162–168. doi: 10.1128/iai.41.1.162-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., Chau G., Goodlin R., Abrams S., Tustian D., Madapallimattam G. The role of human salivary acidic proline-rich proteins in the formation of acquired dental pellicle in vivo and their fate after adsorption to the human enamel surface. Arch Oral Biol. 1983;28(1):19–27. doi: 10.1016/0003-9969(83)90022-5. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Douglas C. W. The binding of human salivary alpha-amylase by oral strains of streptococcal bacteria. Arch Oral Biol. 1983;28(7):567–573. doi: 10.1016/0003-9969(83)90003-1. [DOI] [PubMed] [Google Scholar]
- Eggert F. M. The nature of secretory agglutinins and aggregating factors. I. Secretory conglutinin-like factor, secretory bacterial aggregating factors and secretory IgA antibody in human saliva and amniotic fluid. Int Arch Allergy Appl Immunol. 1980;61(2):192–202. doi: 10.1159/000232433. [DOI] [PubMed] [Google Scholar]
- Eggert F. M. The nature of secretory agglutinins and aggregating factors. II. Biochemical and immunochemical properties of factors in human saliva and amniotic fluid. Int Arch Allergy Appl Immunol. 1980;61(2):203–212. doi: 10.1159/000232434. [DOI] [PubMed] [Google Scholar]
- Eggert F. M. The nature of secretory agglutinins and aggregating factors. III. Secretory conglutinin-like factor SKF detects a cross-reaction between bacteria and complement component C3. Int Arch Allergy Appl Immunol. 1980;62(1):34–45. [PubMed] [Google Scholar]
- Ericson T. Adsorption to hydroxyapatite of proteins and conjugated proteins from human saliva. Caries Res. 1967;1(1):52–58. doi: 10.1159/000259499. [DOI] [PubMed] [Google Scholar]
- Ericson T., Magnusson I. Affinity for hydroxyapatite of salivary substances inducing aggregation of oral streptococci. Caries Res. 1976;10(1):8–18. doi: 10.1159/000260185. [DOI] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Gahnberg L., Olsson J., Krasse B., Carlén A. Interference of Salivary immunoglobulin A antibodies and other salivary fractions with adherence of Streptococcus mutans to hydroxyapatite. Infect Immun. 1982 Aug;37(2):401–406. doi: 10.1128/iai.37.2.401-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Albumin as a blocking agent in studies of streptococcal adsorption to experimental salivary pellicles. Infect Immun. 1985 Nov;50(2):592–594. doi: 10.1128/iai.50.2.592-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res. 1989 Sep;68(9):1303–1307. doi: 10.1177/00220345890680090201. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The interaction of human parotid salivary proteins with hydroxyapatite. Arch Oral Biol. 1973 Dec;18(12):1517–1529. doi: 10.1016/0003-9969(73)90127-1. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Embery G. The isolation and partial characterization of a sulphated glycoprotein from human whole saliva which aggregates strains of Streptococcus sanguis but not Streptococcus mutans. Arch Oral Biol. 1979;24(10-11):791–797. doi: 10.1016/0003-9969(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Kousvelari E. E., Baratz R. S., Burke B., Oppenheim F. G. Immunochemical identification and determination of proline-rich proteins in salivary secretions, enamel pellicle, and glandular tissue specimens. J Dent Res. 1980 Aug;59(8):1430–1438. doi: 10.1177/00220345800590081201. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Michalek S. M. Immunobiology of dental caries: microbial aspects and local immunity. Annu Rev Microbiol. 1981;35:595–638. doi: 10.1146/annurev.mi.35.100181.003115. [DOI] [PubMed] [Google Scholar]
- Moreno E. C., Kresak M., Hay D. I. Adsorption of molecules of biological interest onto hydroxyapatite. Calcif Tissue Int. 1984 Jan;36(1):48–59. doi: 10.1007/BF02405293. [DOI] [PubMed] [Google Scholar]
- Moreno E. C., Kresak M., Hay D. I. Adsorption thermodynamics of acidic proline-rich human salivary proteins onto calcium apatites. J Biol Chem. 1982 Mar 25;257(6):2981–2989. [PubMed] [Google Scholar]
- Morris E. J., McBride B. C. Aggregation of Streptococcus sanguis by a neuraminidase-sensitive component of serum and crevicular fluid. Infect Immun. 1983 Dec;42(3):1073–1080. doi: 10.1128/iai.42.3.1073-1080.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nonaka H. [The effect of bacterial aggregating factor isolated from human whole saliva on adherence of oral Streptococci to hydroxyapatite]. Osaka Daigaku Shigaku Zasshi. 1982 Dec;27(2):168–185. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G. Preliminary observations on the presence and origin of serum albumin in human saliva. Helv Odontol Acta. 1970 Apr;14(1):10–17. [PubMed] [Google Scholar]
- Prakobphol A., Levine M. J., Tabak L. A., Reddy M. S. Purification of a low-molecular-weight, mucin-type glycoprotein from human submandibular-sublingual saliva. Carbohydr Res. 1982 Oct 1;108(1):111–122. doi: 10.1016/s0008-6215(00)81896-0. [DOI] [PubMed] [Google Scholar]
- Rosan B., Appelbaum B., Golub E., Malamud D., Mandel I. D. Enhanced saliva-mediated bacterial aggregation and decreased bacterial adhesion in caries-resistant versus caries-susceptible individuals. Infect Immun. 1982 Dec;38(3):1056–1059. doi: 10.1128/iai.38.3.1056-1059.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Malamud D., Appelbaum B., Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundegren J. L., Arnold R. R. Bacteria-agglutinating characteristics of secretory IgA and a salivary agglutinin. Adv Exp Med Biol. 1987;216B:1005–1013. [PubMed] [Google Scholar]
- Rundegren J., Arnold R. R. Differentiation and interaction of secretory immunoglobulin A and a calcium-dependent parotid agglutinin for several bacterial strains. Infect Immun. 1987 Feb;55(2):288–292. doi: 10.1128/iai.55.2.288-292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Nagata K., Nakamura R., Tsunemitsu A., Misaki A. Interaction of parotid saliva basic glycoprotein with Streptococcus sanguis ATCC 10557. J Periodontol. 1980 Sep;51(9):499–504. doi: 10.1902/jop.1980.51.9.499. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., King W. F. Immunological features of minor salivary gland saliva. J Clin Immunol. 1987 Nov;7(6):449–455. doi: 10.1007/BF00915054. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Levine M. J., Cavese J. M., Prakobphol A., Murray P. A., Tabak L. A., Reddy M. S. Adherence of Streptococcus sanguis to salivary mucin bound to glass. J Dent Res. 1982 Dec;61(12):1390–1393. doi: 10.1177/00220345820610120101. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Jain N. K., Bryan A. R., Cohen R. E., Monte L. D., Zawacki S., Nancollas G. H., Slomiany A., Slomiany B. L. Adsorption of human salivary mucins to hydroxyapatite. Arch Oral Biol. 1985;30(5):423–427. doi: 10.1016/0003-9969(85)90070-6. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- van Houte J. Bacterial specificity in the etiology of dental caries. Int Dent J. 1980 Dec;30(4):305–326. [PubMed] [Google Scholar]