Abstract
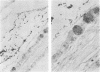
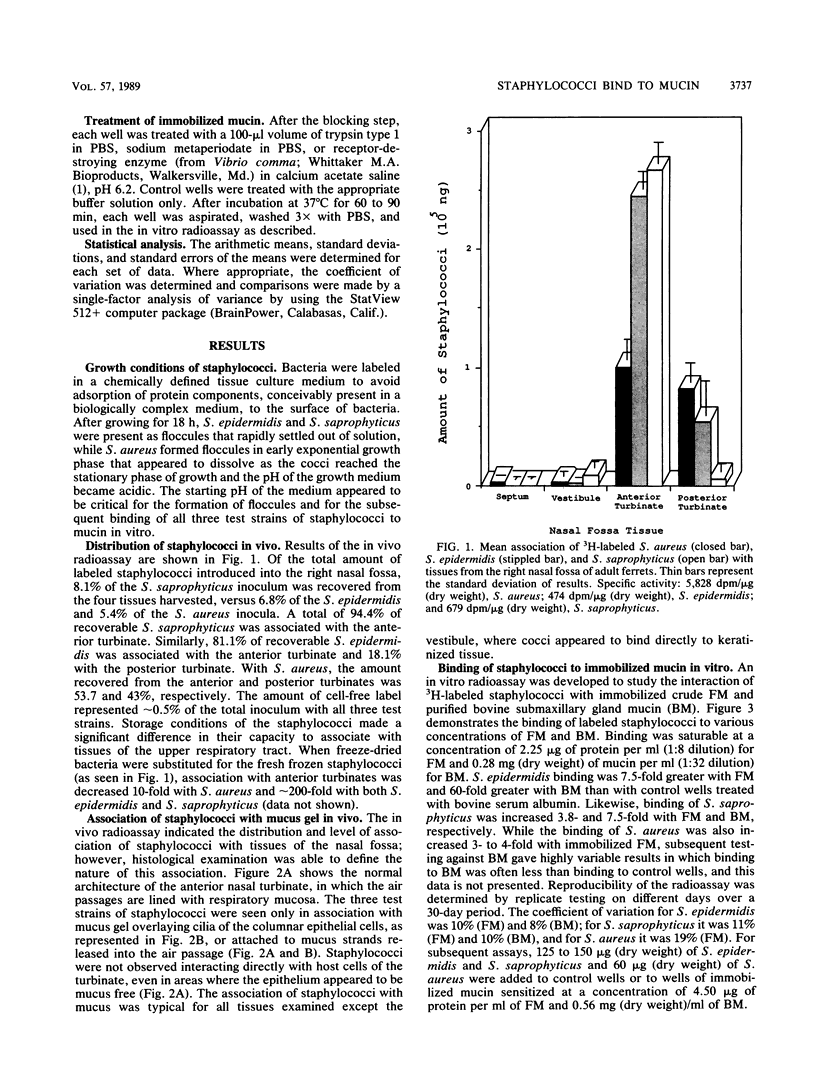
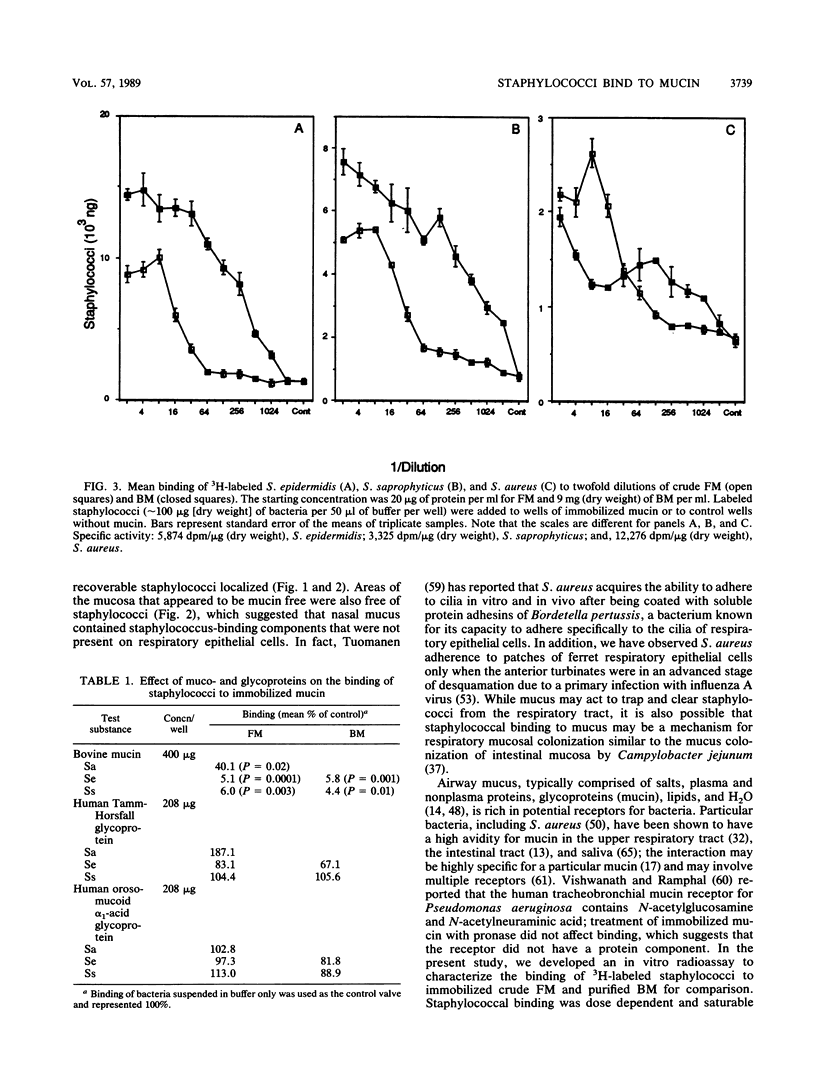
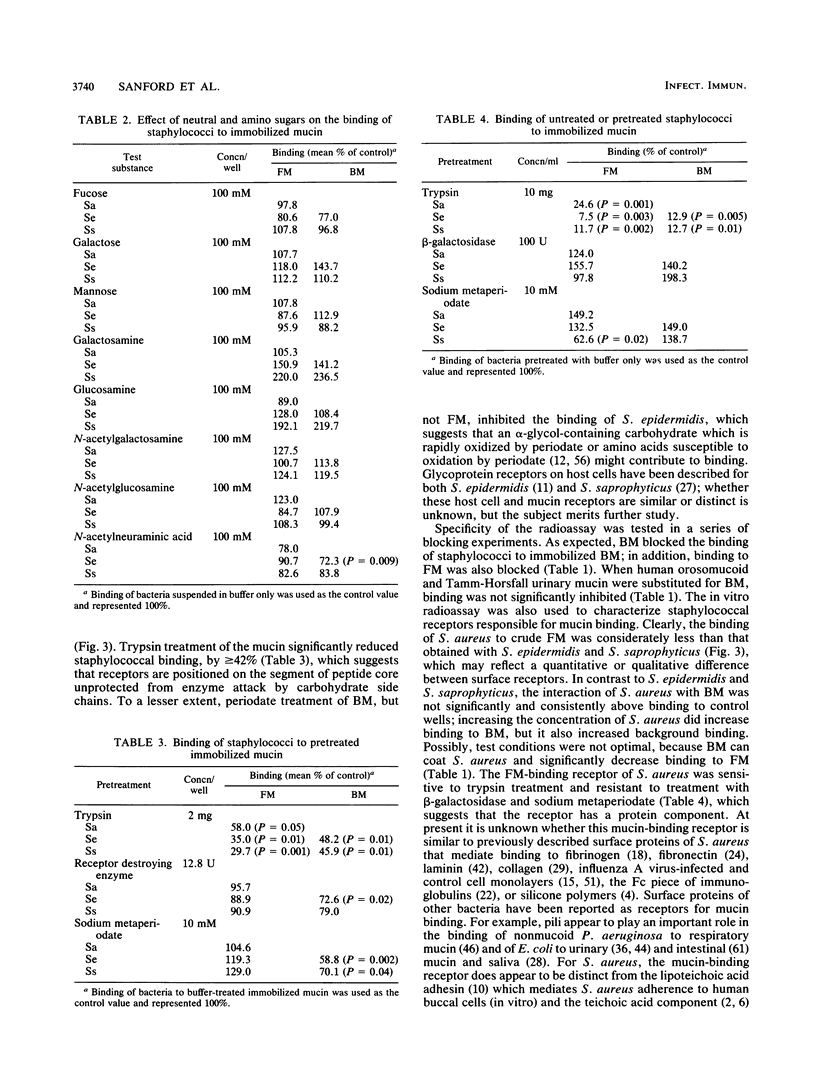
The association of Staphylococcus aureus, Staphylococcus epidermidis, and Staphylococcus saprophyticus with tissues of the upper respiratory tract were compared by using an in vivo ferret model. Ferrets were challenged intranasally with a 1-ml volume of radiolabeled staphylococci (3 mg [dry weight]), were allowed to clear the bacteria in vivo for 90 min, and were sacrificed. Tissues from the right nasal fossa were harvested and processed for radioassay or histology. Of the recoverable staphylococci, greater than or equal to 96% was associated with mucus gel overlaying mucosa of the turbinates. A quantitative radioassay was developed to study the binding of labeled staphylococci to immobilized crude ferret nasal mucin (FM) and bovine submaxillary gland mucin (BM). Binding showed saturation kinetics and was blocked specifically by BM but not by human Tamm-Horsfall glycoprotein nor orosomucoid. Binding to both FM and BM was significantly inhibited (P less than or equal to 0.01) when cocci were pretreated with trypsin but not when treated with beta-galactosidase or sodium metaperiodate (except for binding of S. saprophyticus to FM). These results suggest that mucin-binding receptors of the cocci may have protein components. The staphylococcus-binding receptors of both FM and BM appear to contain protein components, based on sensitivity to pretreatment with trypsin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., STONE J. D. Electrophoretic studies of virus-red cell interaction: mobility gradient of cells treated with viruses of the influenza group and the receptor-destroying enzyme of V. cholerae. Br J Exp Pathol. 1950 Jun;31(3):263–274. [PMC free article] [PubMed] [Google Scholar]
- Aly R., Shinefield H. R., Litz C., Maibach H. I. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J Infect Dis. 1980 Apr;141(4):463–465. doi: 10.1093/infdis/141.4.463. [DOI] [PubMed] [Google Scholar]
- Barrett S. P. Protein-mediated adhesion of Staphylococcus aureus to silicone implant polymer. J Med Microbiol. 1985 Oct;20(2):249–253. doi: 10.1099/00222615-20-2-249. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Bertele R. M., Harms K., Hörl G., Jungwirth R., Petermüller C., Przyklenk B., Weisslein-Pfister C. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection. 1987 Jul-Aug;15(4):270–277. doi: 10.1007/BF01644137. [DOI] [PubMed] [Google Scholar]
- Bibel D. J., Aly R., Shinefield H. R., Maibach H. I., Strauss W. G. Importance of the keratinized epithelial cell in bacterial adherence. J Invest Dermatol. 1982 Oct;79(4):250–253. doi: 10.1111/1523-1747.ep12500072. [DOI] [PubMed] [Google Scholar]
- Bibel D. J., Aly R., Shinefield H. R., Maibach H. I. The Staphylococcus aureus receptor for fibronectin. J Invest Dermatol. 1983 Jun;80(6):494–496. doi: 10.1111/1523-1747.ep12535001. [DOI] [PubMed] [Google Scholar]
- Blum R. A., Rodvold K. A. Recognition and importance of Staphylococcus epidermidis infections. Clin Pharm. 1987 Jun;6(6):464–475. [PubMed] [Google Scholar]
- Burns M. W., May J. R. Bacterial precipitins in serum of patients with cystic fibrosis. Lancet. 1968 Feb 10;1(7537):270–272. doi: 10.1016/s0140-6736(68)90121-9. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers M. M., Kabat W. J. Mediation of staphylococcal adherence to mucosal cells by lipoteichoic acid. Infect Immun. 1983 Apr;40(1):444–446. doi: 10.1128/iai.40.1.444-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M. Constituents of mucus and their separation. Br Med Bull. 1978 Jan;34(1):17–24. doi: 10.1093/oxfordjournals.bmb.a071454. [DOI] [PubMed] [Google Scholar]
- DUTHIE E. S. The action of fibrinogen on certain pathogenic cocci. J Gen Microbiol. 1955 Oct;13(2):383–393. doi: 10.1099/00221287-13-2-383. [DOI] [PubMed] [Google Scholar]
- Davison V. E., Sanford B. A. Adherence of staphylococcus aureus to influenza A virus-infected Madin-Darby canine kidney cell cultures. Infect Immun. 1981 Apr;32(1):118–126. doi: 10.1128/iai.32.1.118-126.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P., Cherry J. D. Scanning electron microscopic demonstration of goblet cell discharge and mucous layer on nasal ciliated respiratory epithelium. Otolaryngol Head Neck Surg. 1980 Jul-Aug;88(4):439–441. [PubMed] [Google Scholar]
- Dulawa J., Jann K., Thomsen M., Rambausek M., Ritz E. Tamm Horsfall glycoprotein interferes with bacterial adherence to human kidney cells. Eur J Clin Invest. 1988 Feb;18(1):87–91. doi: 10.1111/j.1365-2362.1988.tb01171.x. [DOI] [PubMed] [Google Scholar]
- FLOREY H. Mucin and the protection of the body. Proc R Soc Lond B Biol Sci. 1955 Jan 27;143(911):147–158. doi: 10.1098/rspb.1955.0001. [DOI] [PubMed] [Google Scholar]
- Fletcher A. P., Neuberger A., Ratcliffe W. A. Tamm-Horsfall urinary glycoprotein. The chemical composition. Biochem J. 1970 Nov;120(2):417–424. doi: 10.1042/bj1200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Glasgow L. Interaction of viruses and bacteria in host-parasite relations. N Engl J Med. 1972 Jul 6;287(1):42–43. doi: 10.1056/NEJM197207062870111. [DOI] [PubMed] [Google Scholar]
- Gristina A. G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987 Sep 25;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- Gunnarsson A., Mårdh P. A., Lundblad A., Svensson S. Oligosaccharide structures mediating agglutination of sheep erythrocytes by Staphylococcus saprophyticus. Infect Immun. 1984 Jul;45(1):41–46. doi: 10.1128/iai.45.1.41-46.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Simpson W. A. Effects of fibronectin and other salivary macromolecules on the adherence of Escherichia coli to buccal epithelial cells. Infect Immun. 1987 Sep;55(9):2103–2109. doi: 10.1128/iai.55.9.2103-2109.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderbaum D., Hall G. S., Ehrhart L. A. Collagen binding to Staphylococcus aureus. Infect Immun. 1986 Nov;54(2):359–364. doi: 10.1128/iai.54.2.359-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A., Bygren P. Urinary tract infections caused by Staphylococcus saprophyticus: recurrences and complications. J Urol. 1979 Nov;122(5):645–647. doi: 10.1016/s0022-5347(17)56541-6. [DOI] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A. Haemagglutination by Staphylococcus saprophyticus and other staphylococcal species. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):45–50. doi: 10.1111/j.1699-0463.1979.tb02401.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Isayama Y. Evidence for sialyl glycoconjugates as receptors for Bordetella bronchiseptica on swine nasal mucosa. Infect Immun. 1987 Jul;55(7):1607–1609. doi: 10.1128/iai.55.7.1607-1609.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn J. P. Bacterial content and ionic composition of sputum in cystic fibrosis. Lancet. 1978 Feb 11;1(8059):334–334. doi: 10.1016/s0140-6736(78)90111-3. [DOI] [PubMed] [Google Scholar]
- Kloos W. E. Natural populations of the genus Staphylococcus. Annu Rev Microbiol. 1980;34:559–592. doi: 10.1146/annurev.mi.34.100180.003015. [DOI] [PubMed] [Google Scholar]
- Kulczycki L. L., Murphy T. M., Bellanti J. A. Pseudomonas colonization in cystic fibrosis. A study of 160 patients. JAMA. 1978 Jul 7;240(1):30–34. [PubMed] [Google Scholar]
- Kuriyama S. M., Silverblatt F. J. Effect of Tamm-Horsfall urinary glycoprotein on phagocytosis and killing of type I-fimbriated Escherichia coli. Infect Immun. 1986 Jan;51(1):193–198. doi: 10.1128/iai.51.1.193-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., O'Rourke J. L., Barrington P. J., Trust T. J. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect Immun. 1986 Feb;51(2):536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell R. Importance of coagulase-negative staphylococci as pathogens in the urinary tract. Lancet. 1974 Jun 8;1(7867):1155–1158. doi: 10.1016/s0140-6736(74)90634-5. [DOI] [PubMed] [Google Scholar]
- McNabb P. C., Tomasi T. B. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol. 1981;35:477–496. doi: 10.1146/annurev.mi.35.100181.002401. [DOI] [PubMed] [Google Scholar]
- Mota G. F., Carneiro C. R., Gomes L., Lopes J. D. Monoclonal antibodies to Staphylococcus aureus laminin-binding proteins cross-react with mammalian cells. Infect Immun. 1988 Jun;56(6):1580–1584. doi: 10.1128/iai.56.6.1580-1584.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Ramphal R., Guay C., Pier G. B. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. doi: 10.1128/iai.55.3.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P. Chemical aspects of respiratory mucus. Br Med Bull. 1978 Jan;34(1):39–41. [PubMed] [Google Scholar]
- Roberts G. P. Isolation and characterisation of glycoproteins from sputum. Eur J Biochem. 1974 Dec 16;50(1):265–280. doi: 10.1111/j.1432-1033.1974.tb03895.x. [DOI] [PubMed] [Google Scholar]
- Saggers B. A., Lawson D. Affinity for glycoproteins of bacteria found in the respiratory tract in cystic fibrosis. J Clin Pathol. 1970 Apr;23(3):262–265. doi: 10.1136/jcp.23.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B. A., Davison V. E., Ramsay M. A. Staphylococcus aureus adherence to influenza A virus-infected and control cell cultures: evidence for multiple adhesins. Proc Soc Exp Biol Med. 1986 Jan;181(1):104–111. doi: 10.3181/00379727-181-42230. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Ramsay M. A. Bacterial adherence to the upper respiratory tract of ferrets infected with influenza A virus. Proc Soc Exp Biol Med. 1987 Jun;185(2):120–128. doi: 10.3181/00379727-185-42525. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Ramsay M. A. In vivo localization of Staphylococcus aureus in nasal tissues of healthy and influenza A virus-infected ferrets. Proc Soc Exp Biol Med. 1989 Jun;191(2):163–169. doi: 10.3181/00379727-191-42903. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Thomas V. L., Ramsay M. A., Jones T. O. Characterization of clinical strains of Staphylococcus aureus associated with pneumonia. J Clin Microbiol. 1986 Jul;24(1):131–136. doi: 10.1128/jcm.24.1.131-136.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S. W., Adler J. L., Sullivan R. J., Jr, Marine W. M. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968-1969. Arch Intern Med. 1971 Jun;127(6):1037–1041. [PubMed] [Google Scholar]
- Teti G., Chiofalo M. S., Tomasello F., Fava C., Mastroeni P. Mediation of Staphylococcus saprophyticus adherence to uroepithelial cells by lipoteichoic acid. Infect Immun. 1987 Mar;55(3):839–842. doi: 10.1128/iai.55.3.839-842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V. L., Sanford B. A., Keogh B. S., Triplett R. G. Antibody response to Staphylococcus aureus surface proteins in rabbits with persistent osteomyelitis after treatment with demineralized bone implants. Infect Immun. 1989 Feb;57(2):404–412. doi: 10.1128/iai.57.2.404-412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. Piracy of adhesins: attachment of superinfecting pathogens to respiratory cilia by secreted adhesins of Bordetella pertussis. Infect Immun. 1986 Dec;54(3):905–908. doi: 10.1128/iai.54.3.905-908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wadolkowski E. A., Laux D. C., Cohen P. S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of adhesion to mucosal receptors. Infect Immun. 1988 May;56(5):1036–1043. doi: 10.1128/iai.56.5.1036-1043.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmark G., Arremark I., Telander B. Staphylococcus saprophyticus: a frequent cause of acute urinary tract infection among female outpatients. J Infect Dis. 1978 Dec;138(6):791–797. doi: 10.1093/infdis/138.6.791. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]