Abstract
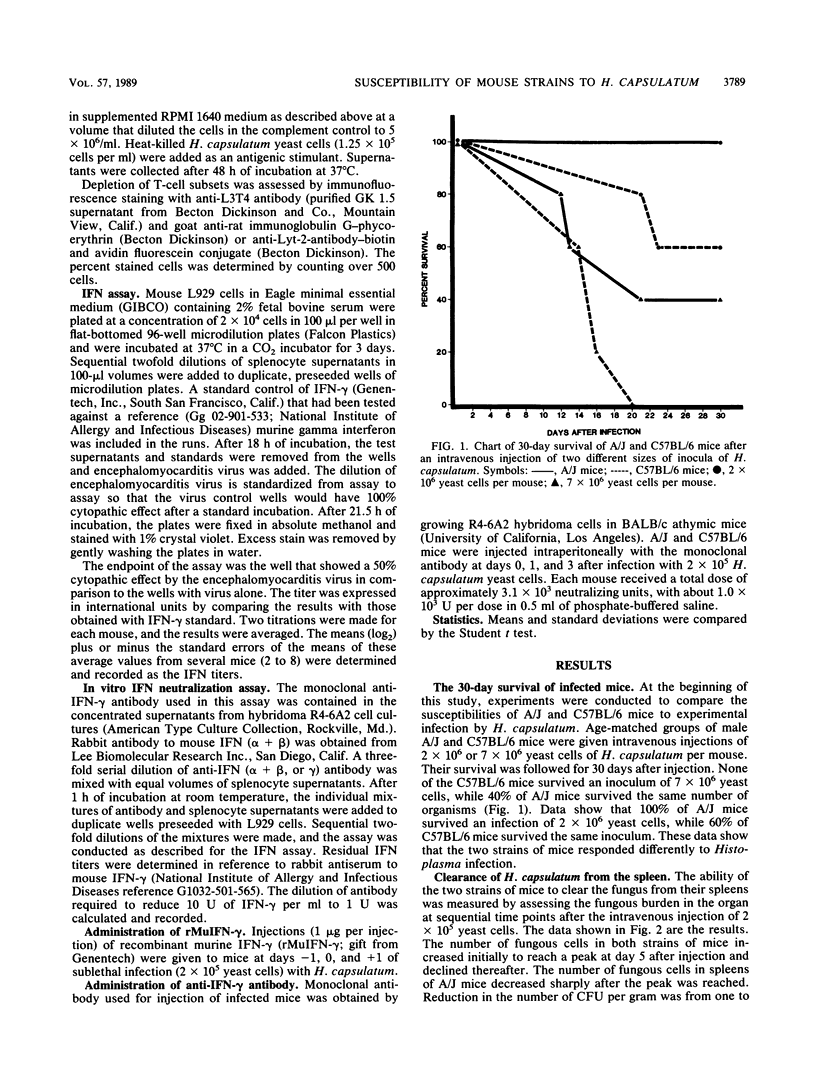
Differences in the 30-day survival of Histoplasma capsulatum after intravenous injection indicated that the A/J strain of inbred mouse was more resistant to experimental infection than was the C57BL/6 strain. CFU from the spleens of infected animals increased during the first week after injection but gradually declined over the next 3 weeks. The CFU per gram of tissue in the C57BL/6 animals were 10- to 100-fold higher than were those in the A/J mice during the time between 7 and 28 days after infection. The units of gamma interferon (IFN-gamma) in supernatants of spleen cells stimulated with heat-killed yeast cells of H. capsulatum reached a peak at the time of the largest number of CFU per gram of tissue. The titers of IFN-gamma at days 3 to 5 were higher in the A/J mice than they were in the C57BL/6 mice, but from days 7 to 28, the titers of IFN-gamma were not correlated with the more efficient clearance of the fungus from the spleens of A/J mice. The L3T4+ spleen cells were shown to be active IFN-gamma producers. Treatment of Histoplasma-infected mice with anti-IFN-gamma antibody resulted in much larger tissue burdens of the fungus in the lungs and spleens of treated animals than in untreated animals. There was no marked difference in the result of treatment with anti-IFN-gamma antibody between A/J and C57BL/6 mice. Treatment of Histoplasma-infected mice with recombinant murine IFN-gamma did not alter the course of infection in either inbred strain of mouse.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effectory responses by activation of splenic suppressor cells. Infect Immun. 1979 Mar;23(3):893–902. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. L. The production of disseminated histoplasmosis in the mouse: the eff4ects of changes in reticulo-endothelial function. J Pathol. 1969 Mar;97(3):441–457. doi: 10.1002/path.1710970304. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Friedman H., Stewart W. E., 2nd, Klein T. W., Djeu J. Y. Role of gamma interferon in induction of natural killer activity by Legionella pneumophila in vitro and in an experimental murine infection model. Infect Immun. 1988 May;56(5):1187–1193. doi: 10.1128/iai.56.5.1187-1193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick E. W., Roberts G. D. The varying susceptibility of different genetic strains of laboratory mice to Histoplasma capsulatum. Mycopathol Mycol Appl. 1974 Apr 30;52(3):251–253. doi: 10.1007/BF02198750. [DOI] [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V., Hoffman M. Clearance and distribution of recombinant murine gamma-interferon in mice. Cancer Res. 1988 Apr 15;48(8):2021–2024. [PubMed] [Google Scholar]
- HOWELL A., Jr, KIPKIE G. F. A comparison of the susceptibility by intracerebral inoculation of six strains of mice with male DBA line 1 mice. Am J Trop Med Hyg. 1951 Jan;31(1):33–41. doi: 10.4269/ajtmh.1951.s1-31.33. [DOI] [PubMed] [Google Scholar]
- Howard D. H. Further studies on the inhibition of Histoplasma capsulatum within macrophages from immunized animals. Infect Immun. 1973 Oct;8(4):577–581. doi: 10.1128/iai.8.4.577-581.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V., Gupta R. K. Lymphocyte-mediated cellular immunity in histoplasmosis. Infect Immun. 1971 Nov;4(5):605–610. doi: 10.1128/iai.4.5.605-610.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Jerrells T. R., Spitalny G. L., Walker D. H. Gamma interferon as a crucial host defense against Rickettsia conorii in vivo. Infect Immun. 1987 May;55(5):1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R., Salvin S. B., Shreffler D. C. Mechanisms in the in vivo release of lymphokines. VI. Studies with mice congenic at the H-2 region. Transplantation. 1982 Jan;33(1):102–103. doi: 10.1097/00007890-198201000-00022. [DOI] [PubMed] [Google Scholar]
- Nickerson D. A., Havens R. A., Bullock W. E. Immunoregulation in disseminated histoplasmosis: characterization of splenic suppressor cell populations. Cell Immunol. 1981 May 15;60(2):287–297. doi: 10.1016/0008-8749(81)90270-7. [DOI] [PubMed] [Google Scholar]
- Palmer B. A., Hetrick F. M., Jerrells T. J. Production of gamma interferon in mice immune to Rickettsia tsutsugamushi. Infect Immun. 1984 Jan;43(1):59–65. doi: 10.1128/iai.43.1.59-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Bullock W. E. Immunoregulation in disseminated histoplasmosis: characterization of the surface phenotype of splenic suppressor T lymphocytes. Infect Immun. 1982 Sep;37(3):940–945. doi: 10.1128/iai.37.3.940-945.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B. A., Howard D. H. Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect Immun. 1987 Apr;55(4):1014–1016. doi: 10.1128/iai.55.4.1014-1016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H., Ahmed R. Virus-induced immunosuppression: a murine model of susceptibility to opportunistic infection. J Infect Dis. 1988 Jul;158(1):232–235. doi: 10.1093/infdis/158.1.232. [DOI] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Macrophage cell lines P388D1 and IC-21 stimulated with gamma interferon fail to inhibit the intracellular growth of Histoplasma capsulatum. Infect Immun. 1989 Sep;57(9):2903–2905. doi: 10.1128/iai.57.9.2903-2905.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. M., Peterson E. M., Czarniecki C. W., Schreiber R. D., de la Maza L. M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989 Jan;57(1):152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



