Abstract
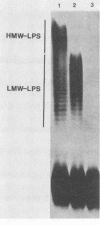
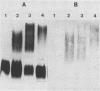
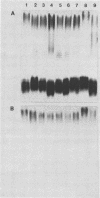
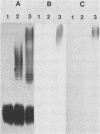
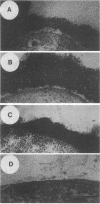
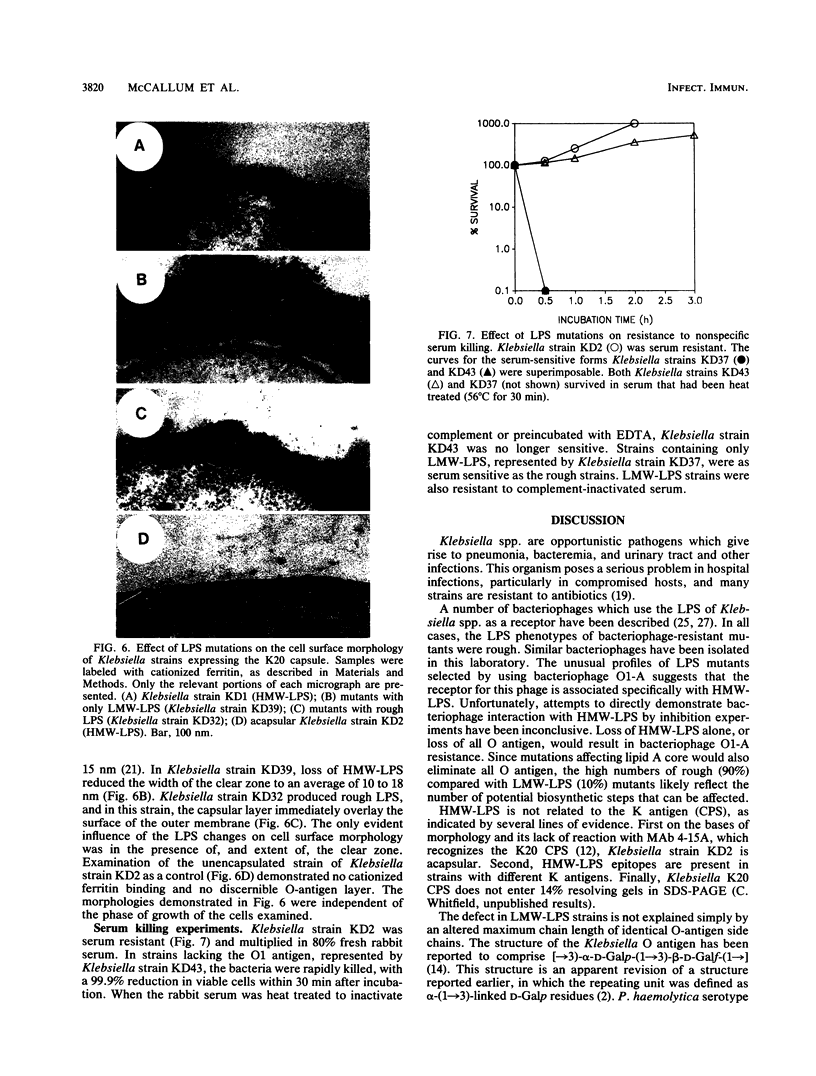
The lipopolysaccharide (LPS) O-antigen side chains of Klebsiella serotype O1 have been studied by using mutants selected by resistance to a Klebsiella bacteriophage designated O1-A. Two classes of LPS mutants were identified. The major group (90%) synthesized rough LPS. The remaining 10% of the mutants produced a novel LPS profile that lacked the highest-molecular-weight O-substituted molecules (HMW-LPS) but still produced lower-molecular-weight O-substituted species (LMW-LPS). By using antisera raised against mutant Klebsiella strains and antiserum specific for Pasteurella haemolytica serotype 4, it was demonstrated that HMW-LPS and LMW-LPS contain shared epitopes. HMW-LPS also contained an epitope absent in LMW-LPS. This unique epitope was recognized by a monoclonal antibody (O1-52.6) and appears to be responsible for the serological cross-reaction between the O antigens of Klebsiella O1 and Escherichia coli O19. This HMW-LPS epitope was present in eight other Klebsiella O1 isolates which were examined. Electron microscopy demonstrated that HMW-LPS excluded overlying capsular polysaccharide for a distance of 25 to 40 nm. The distance was reduced to 10 to 18 nm in strains which synthesized only LMW-LPS and to zero in rough LPS strains. The HMW-LPS of Klebsiella O1 was shown to be an important virulence determinant, since this molecule was responsible for the resistance of the bacterium to nonspecific, complement-mediated serum killing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björndal H., Lindberg B., Nimmich W. Structural studies on Klebsiella O groups 1 and 6 lipopolysaccharides. Acta Chem Scand. 1971;25(2):750–750. doi: 10.3891/acta.chem.scand.25-0750. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Joiner K., Hammer C. The function of antibody and complement in the lysis of bacteria. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S537–S545. doi: 10.1093/clinids/9.supplement_5.s537. [DOI] [PubMed] [Google Scholar]
- Galili U., Mandrell R. E., Hamadeh R. M., Shohet S. B., Griffiss J. M. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988 Jul;56(7):1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N., Schmetz M. A., Foulds J., Klima E. N., Jimenez-Lucho V. E., Leive L. L., Joiner K. A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987 Feb;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homonylo M. K., Wilmot S. J., Lam J. S., MacDonald L. A., Whitfield C. Monoclonal antibodies against the capsular K antigen of Escherichia coli (O9:K30(A):H12): characterisation and use in analysis of K antigen organisation on the cell surface. Can J Microbiol. 1988 Oct;34(10):1159–1165. doi: 10.1139/m88-204. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Grossman N., Schmetz M., Leive L. C3 binds preferentially to long-chain lipopolysaccharide during alternative pathway activation by Salmonella montevideo. J Immunol. 1986 Jan;136(2):710–715. [PubMed] [Google Scholar]
- Lam J. S., Lam M. Y., MacDonald L. A., Hancock R. E. Visualization of Pseudomonas aeruginosa O antigens by using a protein A-dextran-colloidal gold conjugate with both immunoglobulin G and immunoglobulin M monoclonal antibodies. J Bacteriol. 1987 Aug;169(8):3531–3538. doi: 10.1128/jb.169.8.3531-3538.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J Immunol. 1983 Apr;130(4):1867–1870. [PubMed] [Google Scholar]
- Mizuta K., Ohta M., Mori M., Hasegawa T., Nakashima I., Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983 Apr;40(1):56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomerie J. Z. Epidemiology of Klebsiella and hospital-associated infections. Rev Infect Dis. 1979 Sep-Oct;1(5):736–753. doi: 10.1093/clinids/1.5.736. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás J. M., Benedí V. J., Ciurana B., Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986 Oct;54(1):85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás J. M., Ciurana B., Benedí V. J., Juarez A. Role of lipopolysaccharide and complement in susceptibility of Escherichia coli and Salmonella typhimurium to non-immune serum. J Gen Microbiol. 1988 Apr;134(4):1009–1016. doi: 10.1099/00221287-134-4-1009. [DOI] [PubMed] [Google Scholar]
- Tomás J. M., Jofre J. T. Lipopolysaccharide-specific bacteriophage for Klebsiella pneumoniae C3. J Bacteriol. 1985 Jun;162(3):1276–1279. doi: 10.1128/jb.162.3.1276-1279.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]