Abstract
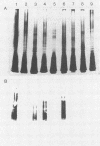
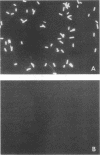
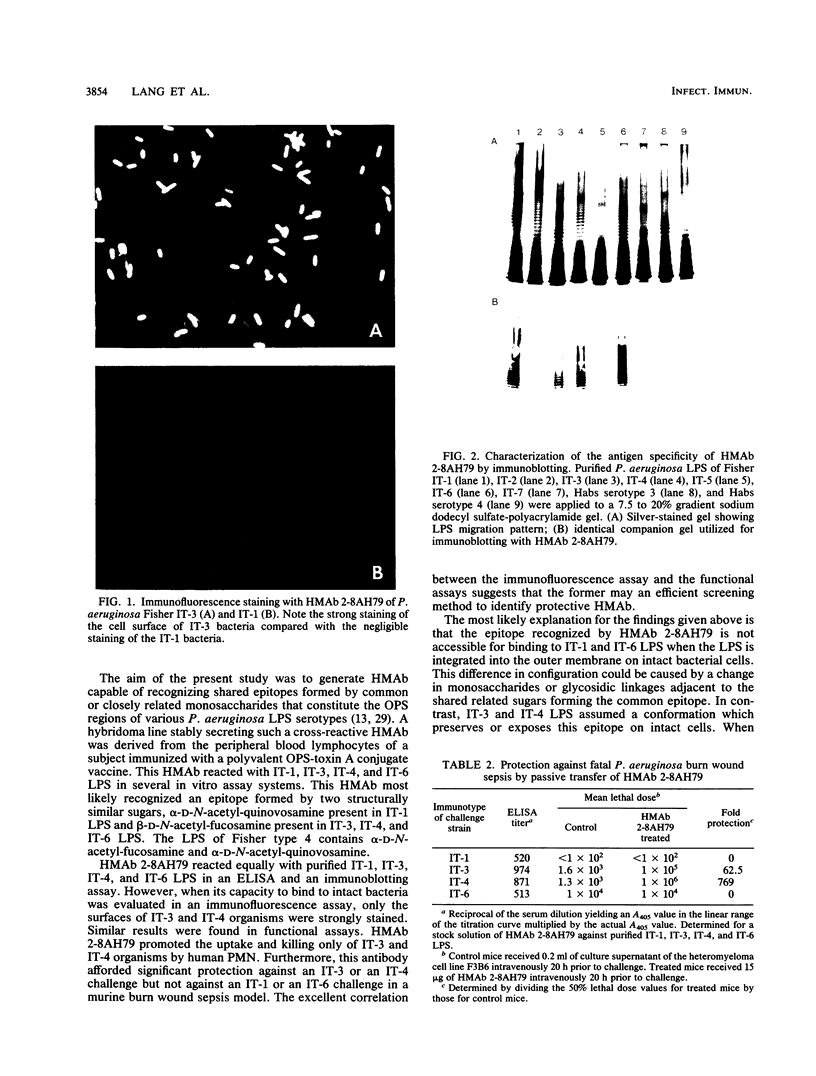
A hybridoma line secreting a human monoclonal antibody (HMAb) capable of recognizing Fisher immunotype (IT) 1, 3, 4, and 6 lipopolysaccharide (LPS) in vitro was isolated. Peripheral blood lymphocytes (PBL) were obtained from volunteers immunized with an experimental Pseudomonas aeruginosa O polysaccharide-toxin A vaccine. PBL-expressing surface antibodies able to bind to P. aeruginosa LPS were isolated by adsorption onto LPS-coated plastic wells. Such PBL were transformed with Epstein-Barr virus. Lymphoblastoid cell lines secreting anti-P. aeruginosa LPS antibodies were identified by an enzyme-linked immunosorbent assay and fused to the F3B6 heteromyeloma line. A hybridoma line producing a HMAb (2-8AH79) able to bind IT-1, IT-3, IT-4, and IT-6 LPS was identified by an enzyme-linked immunosorbent assay. This HMAb was found to bind to the O-polysaccharide regions of IT-1, IT-3, IT-4, and IT-6 LPS, as determined by immunoblotting. By using an immunofluorescence microscopy assay, the cell surfaces of IT-3 and IT-4 bacteria were strongly stained by HMAb 2-8AH79, whereas those of IT-1 and IT-6 bacteria were weakly stained. This HMAb was found to promote the uptake and killing of IT-3 and IT-4 bacteria, but not IT-1 or IT-6 organisms, by human polymorphonuclear leukocytes. Similarly, the passive transfer of HMAb 2-8AH79 to mice afforded significant protection only against a challenge with IT-3 and IT-4 bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brenner E. R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983 Jul-Aug;5(4):629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- Collins M. S., Roby R. E. Protective activity of an intravenous immune globulin (human) enriched in antibody against lipopolysaccharide antigens of Pseudomonas aeruginosa. Am J Med. 1984 Mar 30;76(3A):168–174. doi: 10.1016/0002-9343(84)90337-1. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Cross A. S., Wegmann A., Germanier R., Sadoff J. C. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987 Jul;80(1):51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Sadoff J. C., Germanier R. A polyvalent Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Antibiot Chemother (1971) 1987;39:249–255. doi: 10.1159/000414350. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foung S. K., Perkins S., Raubitschek A., Larrick J., Lizak G., Fishwild D., Engleman E. G., Grumet F. C. Rescue of human monoclonal antibody production from an EBV-transformed B cell line by fusion to a human-mouse hybridoma. J Immunol Methods. 1984 May 11;70(1):83–90. doi: 10.1016/0022-1759(84)90392-2. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Roe E. A., Gupta J. L. Controlled trial of Pseudomonas immunoglobulin and vaccine in burn patients. Lancet. 1980 Dec 13;2(8207):1263–1265. doi: 10.1016/s0140-6736(80)92334-x. [DOI] [PubMed] [Google Scholar]
- King A., Shannon K., Eykyn S., Phillips I. Reduced sensitivity to beta-lactam antibiotics arising during ceftazidime treatment of Pseudomonas aeruginosa infections. J Antimicrob Chemother. 1983 Oct;12(4):363–370. doi: 10.1093/jac/12.4.363. [DOI] [PubMed] [Google Scholar]
- Knirel YuA, Vinogradov E. V., Kocharova N. A., Paramonov N. A., Kochetkov N. K., Dmitriev B. A., Stanislavsky E. S., Lányi B. The structure of O-specific polysaccharides and serological classification of Pseudomonas aeruginosa (a review). Acta Microbiol Hung. 1988;35(1):3–24. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Barnes M. W., Finland M. Bacteremia at Boston City Hospital: Occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis. 1975 Sep;132(3):316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Menkes E. Type-specific vs. cross-protective vaccination for gram-negative bacterial pneumonia. J Infect Dis. 1981 Dec;144(6):599–603. doi: 10.1093/infdis/144.6.599. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. Protective immunity induced in mice by immunization with high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Thomas D. M. Lipopolysaccharide and high-molecular-weight polysaccharide serotypes of Pseudomonas aeruginosa. J Infect Dis. 1982 Feb;145(2):217–223. doi: 10.1093/infdis/145.2.217. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Moellering R. C., Jr, Martin R. R., Perkins R. L., Strike D. G., Gootz T. D., Sanders W. E., Jr Resistance to cefamandole: a collaborative study of emerging clinical problems. J Infect Dis. 1982 Jan;145(1):118–125. doi: 10.1093/infdis/145.1.118. [DOI] [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y. Immunoprotective human monoclonal antibodies against five major serotypes of Pseudomonas aeruginosa. J Gen Microbiol. 1987 Dec;133(12):3581–3590. doi: 10.1099/00221287-133-12-3581. [DOI] [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y., Tomibe K. Characterization of a human monoclonal antibody to lipopolysaccharides of Pseudomonas aeruginosa serotype 5: a possible candidate as an immunotherapeutic agent for infections with P. aeruginosa. J Infect Dis. 1985 Nov;152(5):965–970. doi: 10.1093/infdis/152.5.965. [DOI] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wesley J., Fisher A., Fisher M. W. Immunization against Pseudomonas in infection after thermal injury. J Infect Dis. 1974 Nov;130 (Suppl)(0):S152–S158. doi: 10.1093/infdis/130.supplement.s152. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. Composition and structure of lipopolysaccharides from Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S941–S949. doi: 10.1093/clinids/5.supplement_5.s941. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Gammon M. C., Hutchison C. F., Jackson J. J., Lombardo D., Miner K. M., Puckett J. M., Sewell T. J., Sigal N. H. Human monoclonal antibodies that protect mice against challenge with Pseudomonas aeruginosa. Infect Immun. 1988 Aug;56(8):1873–1879. doi: 10.1128/iai.56.8.1873-1879.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]