Abstract
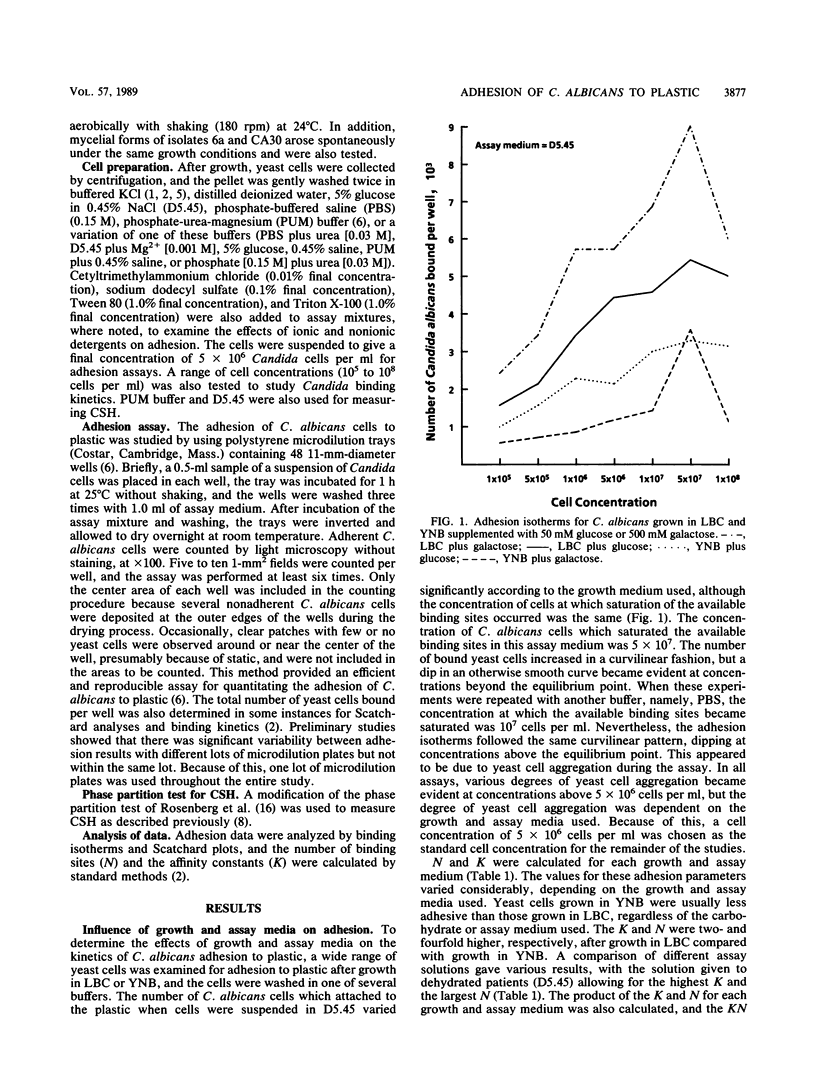
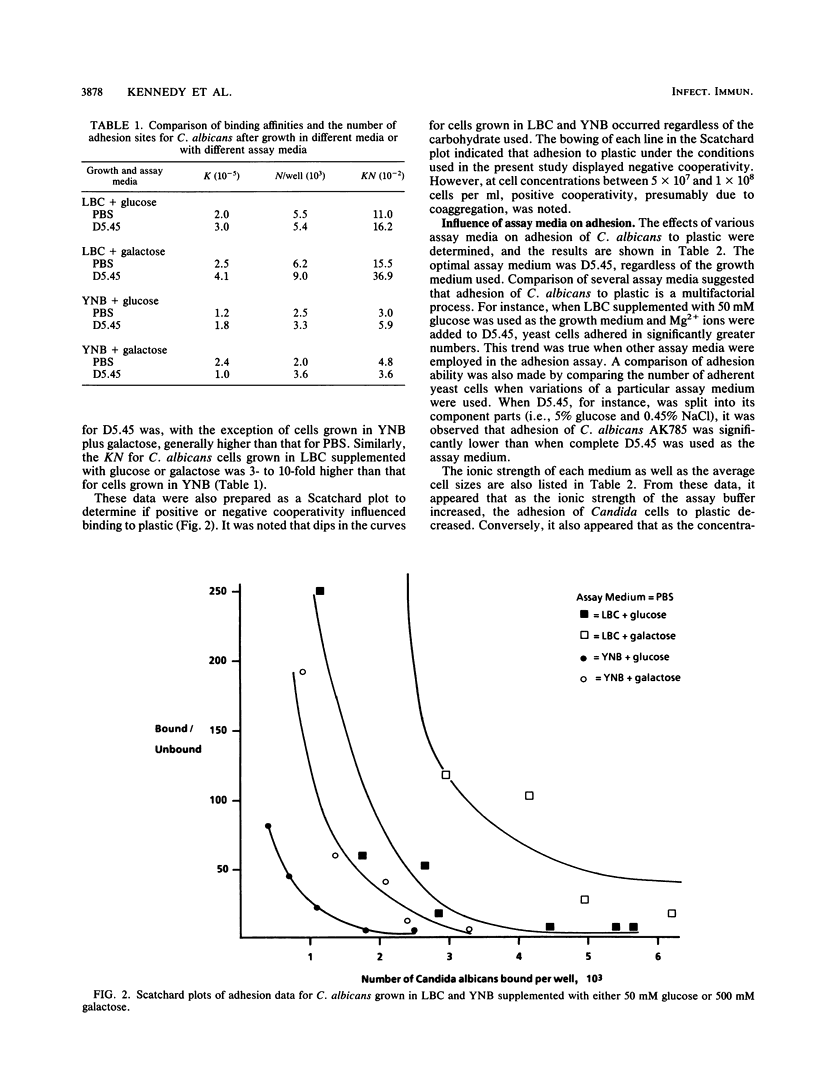
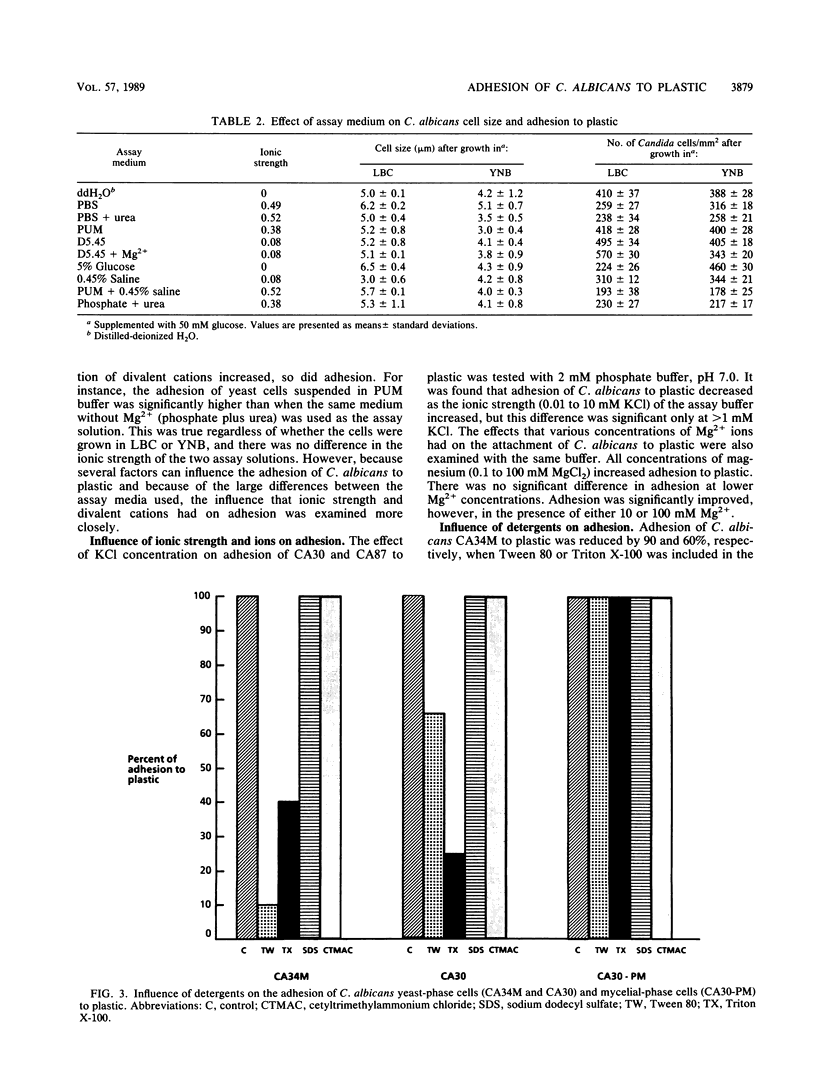
The adhesion of Candida albicans to plastic was examined after growth in two chemically defined media, Lee-Buckley-Campbell (LBC) and yeast nitrogen base (YNB), by binding isotherms, Langmuir isotherms, and Scatchard plots, and the number of binding sites (N) and the affinity constants (K) were calculated. K and N were twofold and fourfold higher, respectively, after growth in LBC compared with that in YNB. A comparison of adhesion in different assay solutions gave similar results, with the solution given to dehydrated patients (5% glucose in 0.45% NaCl [D5.45]) allowing for the highest K and the largest N. Scatchard curves for both LBC- and YNB-grown cells had negative slopes, which is supportive evidence for the view that negative cooperativity is involved in the binding process. Additional experiments to examine the role of cell surface hydrophobicity in adhesion to plastic were conducted with the white and opaque phenotypes of C. albicans. There was no significant difference in the adhesion of these phenotypes to plastic, although the opaque phenotype was significantly more hydrophobic. Adhesion, but not cell surface hydrophobicity, of both phenotypes was significantly greater in D5.45. Moreover, relatively hydrophilic mycelial forms of C. albicans were found to attach only when D5.45 was used as the assay medium and, in contrast to yeast-phase cells, were insensitive to reduced adhesion by nonionic detergents. These results suggest that the adhesion of C. albicans to plastic is regulated by environmental circumstances and the phenotypic state of the organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina A. G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987 Sep 25;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Plotkin B. J., Klimas D. M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect Immun. 1986 Oct;54(1):269–271. doi: 10.1128/iai.54.1.269-271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J. Adhesion and association mechanisms of Candida albicans. Curr Top Med Mycol. 1988;2:73–169. doi: 10.1007/978-1-4612-3730-3_4. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Rogers A. L., Hanselmen L. R., Soll D. R., Yancey R. J., Jr Variation in adhesion and cell surface hydrophobicity in Candida albicans white and opaque phenotypes. Mycopathologia. 1988 Jun;102(3):149–156. doi: 10.1007/BF00437397. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Sandin R. L. Influence of growth conditions on Candida albicans adhesion, hydrophobicity and cell wall ultrastructure. J Med Vet Mycol. 1988 Apr;26(2):79–92. [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A., Edwards C. A., Yancey R. J. Mechanisms of association of Candida albicans with intestinal mucosa. J Med Microbiol. 1987 Dec;24(4):333–341. doi: 10.1099/00222615-24-4-333. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Zajic J. E. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985 Oct;50(1):97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Locci R., Peters G., Pulverer G. Microbial colonization of prosthetic devices. IV. Scanning electron microscopy of intravenous catheters invaded by yeasts. Zentralbl Bakteriol Mikrobiol Hyg B. 1981 Sep;173(6):419–424. [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981 Jun;32(3):1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagi S., Miyake Y., Inagaki K., Tsuru H., Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect Immun. 1985 Jan;47(1):11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y., Fujita Y., Minagi S., Suginaka H. Surface hydrophobicity and adherence of Candida to acrylic surfaces. Microbios. 1986;46(186):7–14. [PubMed] [Google Scholar]
- Rotrosen D., Gibson T. R., Edwards J. E., Jr Adherence of candida species to intravenous catheters. J Infect Dis. 1983 Mar;147(3):594–594. doi: 10.1093/infdis/147.3.594. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


