Abstract
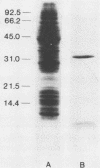
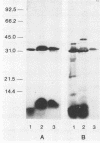
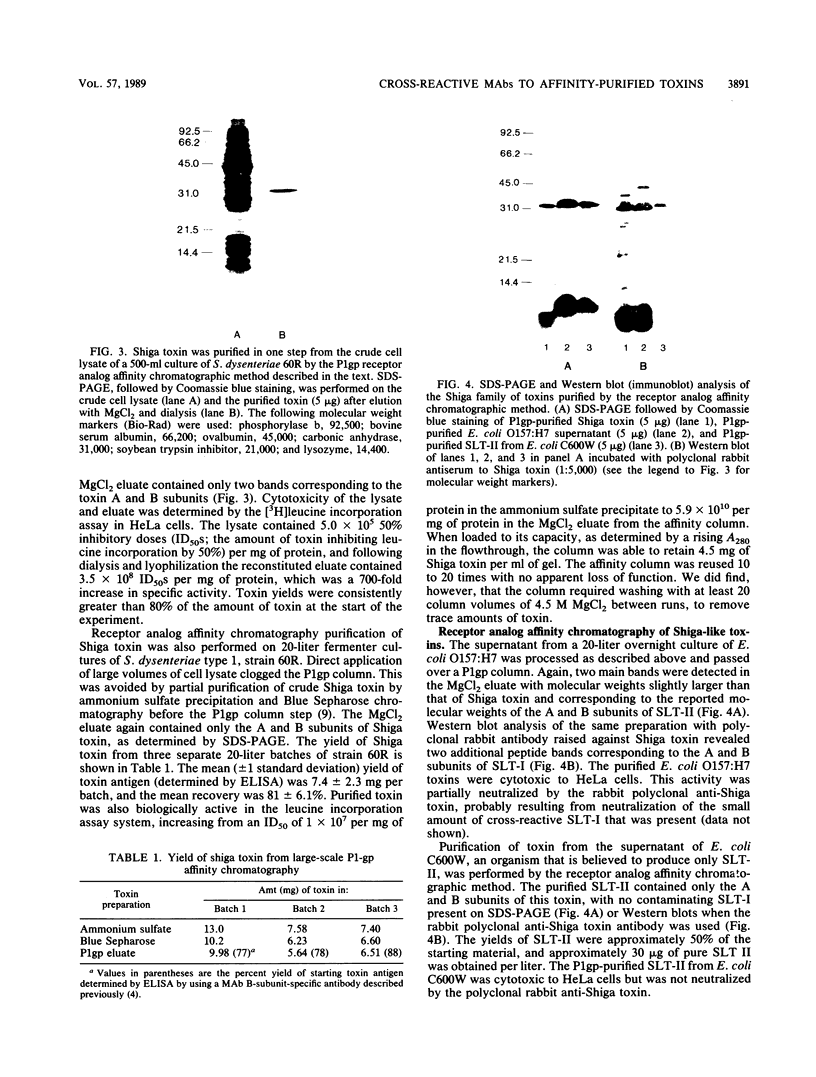
Shiga toxin from Shigella dysenteriae 60R was purified to homogeneity by a novel one-step receptor analog affinity chromatography method. The method was based on the binding affinity of Shiga toxin for a specific disaccharide, Gal alpha 1----4Gal, which was also present in glycoproteins with P1 blood group seroreactivity produced in hydatid cysts from sheep infected with Echinococcus granulosus. Having shown that cyst fluid P1 glycoprotein bound Shiga toxin on a solid phase, a P1 glycoprotein affinity column was made by coupling P1-active substance to Sepharose 4B. Shiga toxin was purified by this method in large quantities (5 to 10 mg/20-liter batch) with a consistently good yield (greater than 80% of starting toxin). Shiga-like toxins I and II (SLT-I and -II, respectively) from Escherichia coli were also purified by the same method. A preparation containing SLT-II and SLT-I purified by receptor analog affinity chromatography was used to raise four monoclonal antibodies (MAbs) that were reactive with SLT-II by enzyme-linked immunosorbent assay. Three of these antibodies also reacted with Shiga toxin, which was the first clear demonstration of cross-reactivity between these toxins. One MAb, 4D1, which was specific for the B subunit of SLT-II and Shiga toxin, neutralized both toxins in a HeLa cell cytotoxicity assay. Two MAbs recognized the A subunit of both SLT-II and Shiga toxin by Western blot (immunoblot) analysis but were unable to neutralize either toxin. In addition, one B-subunit-specific MAb neutralized SLT-II alone, and a previously described Shiga toxin B-subunit-specific MAb was shown to be specific for Shiga toxin but not SLT-II.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Griffin D. E., Rothman S. W., Doctor B. P. Purification and biological characterization of shiga toxin from Shigella dysenteriae 1. Infect Immun. 1982 Jun;36(3):996–1005. doi: 10.1128/iai.36.3.996-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory H. T., Yates A. D., Donald A. S., Watkins W. M., Morgan W. T. The nature of the human blood group P1 determinant. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1289–1296. doi: 10.1016/s0006-291x(74)80424-9. [DOI] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Kelley M. A., Bennish M., Keusch G. T. Enzyme-linked immunosorbent assay for shigella toxin. J Clin Microbiol. 1986 Jul;24(1):65–68. doi: 10.1128/jcm.24.1.65-68.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Keusch G. T., Edson C., Thorley-Lawson D., Jacewicz M. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of Shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J Exp Med. 1984 Dec 1;160(6):1767–1781. doi: 10.1084/jem.160.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes F. P., Barrett T. J., Green J. H., Aloisio C. H., Spika J. S., Strockbine N. A., Wachsmuth I. K. Affinity purification and characterization of Shiga-like toxin II and production of toxin-specific monoclonal antibodies. Infect Immun. 1988 Aug;56(8):1926–1933. doi: 10.1128/iai.56.8.1926-1933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K., Yutsudo T., Takeda Y., Ogasawara T., Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988 Jan 15;171(1-2):45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G. T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986 Jun 1;163(6):1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Donohue-Rolfe A., Jacewicz M., Kane A. V. Shiga toxin: production and purification. Methods Enzymol. 1988;165:152-62, 399-401. doi: 10.1016/s0076-6879(88)65025-7. [DOI] [PubMed] [Google Scholar]
- MORGAN W. T., WATKINS W. M. BLOOD GROUP P1 SUBSTANCE. I. CHEMICAL PROPERTIES. Bibl Haematol. 1964;19:225–229. doi: 10.1159/000426548. [DOI] [PubMed] [Google Scholar]
- Marcus D. M., Kundu S. K., Suzuki A. The P blood group system: recent progress in immunochemistry and genetics. Semin Hematol. 1981 Jan;18(1):63–71. [PubMed] [Google Scholar]
- O'Brien A. D., Chen M. E., Holmes R. K., Kaper J., Levine M. M. Environmental and human isolates of Vibrio cholerae and Vibrio parahaemolyticus produce a Shigella dysenteriae 1 (Shiga)-like cytotoxin. Lancet. 1984 Jan 14;1(8368):77–78. doi: 10.1016/s0140-6736(84)90006-0. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D., Griffin D. E., Thompson M. R. Characterization of Shigella dysenteriae 1 (Shiga) toxin purified by anti-Shiga toxin affinity chromatography. Infect Immun. 1980 Oct;30(1):170–179. doi: 10.1128/iai.30.1.170-179.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Newland J. W., Miller S. F., Holmes R. K., Smith H. W., Formal S. B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984 Nov 9;226(4675):694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- Perera L. P., Marques L. R., O'Brien A. D. Isolation and characterization of monoclonal antibodies to Shiga-like toxin II of enterohemorrhagic Escherichia coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Oct;26(10):2127–2131. doi: 10.1128/jcm.26.10.2127-2131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Jackson M. P., Sung L. M., Holmes R. K., O'Brien A. D. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J Bacteriol. 1988 Mar;170(3):1116–1122. doi: 10.1128/jb.170.3.1116-1122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Newland J. W., Smith H. W., Holmes R. K., O'Brien A. D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986 Jul;53(1):135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell T., Head S., Petric M., Cohen A., Lingwood C. Globotriosyl ceramide is specifically recognized by the Escherichia coli verocytotoxin 2. Biochem Biophys Res Commun. 1988 Apr 29;152(2):674–679. doi: 10.1016/s0006-291x(88)80091-3. [DOI] [PubMed] [Google Scholar]