Abstract
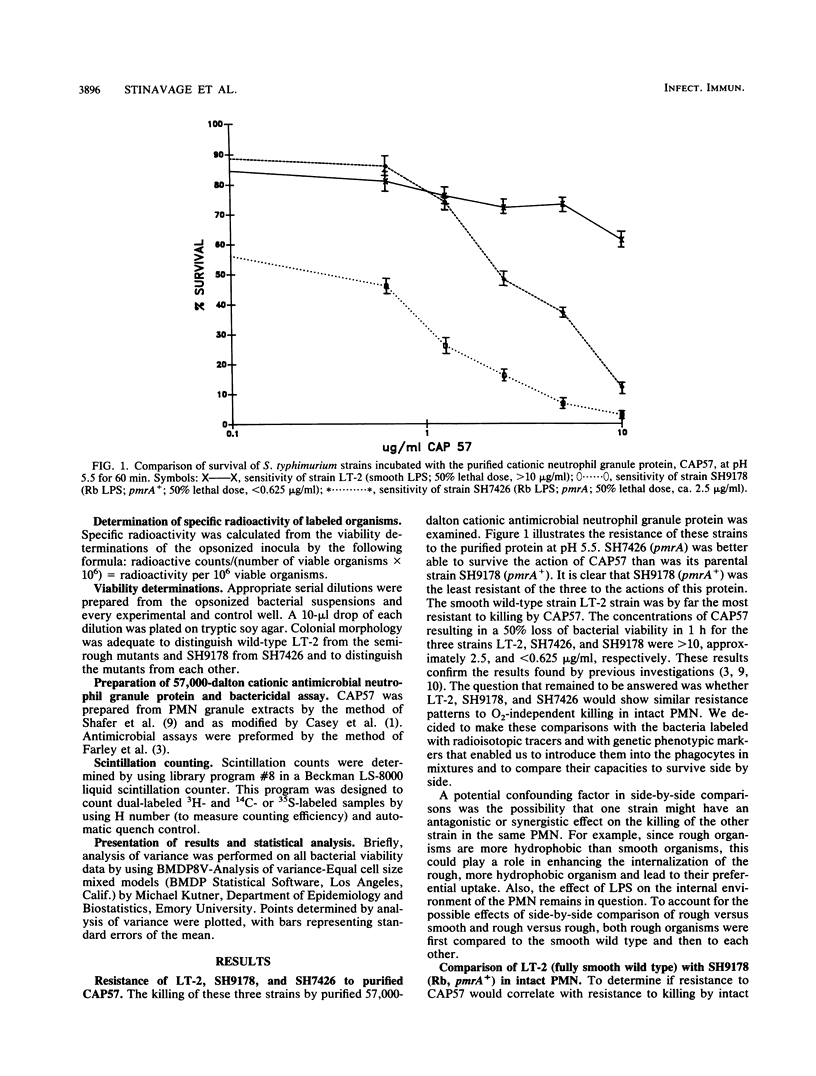
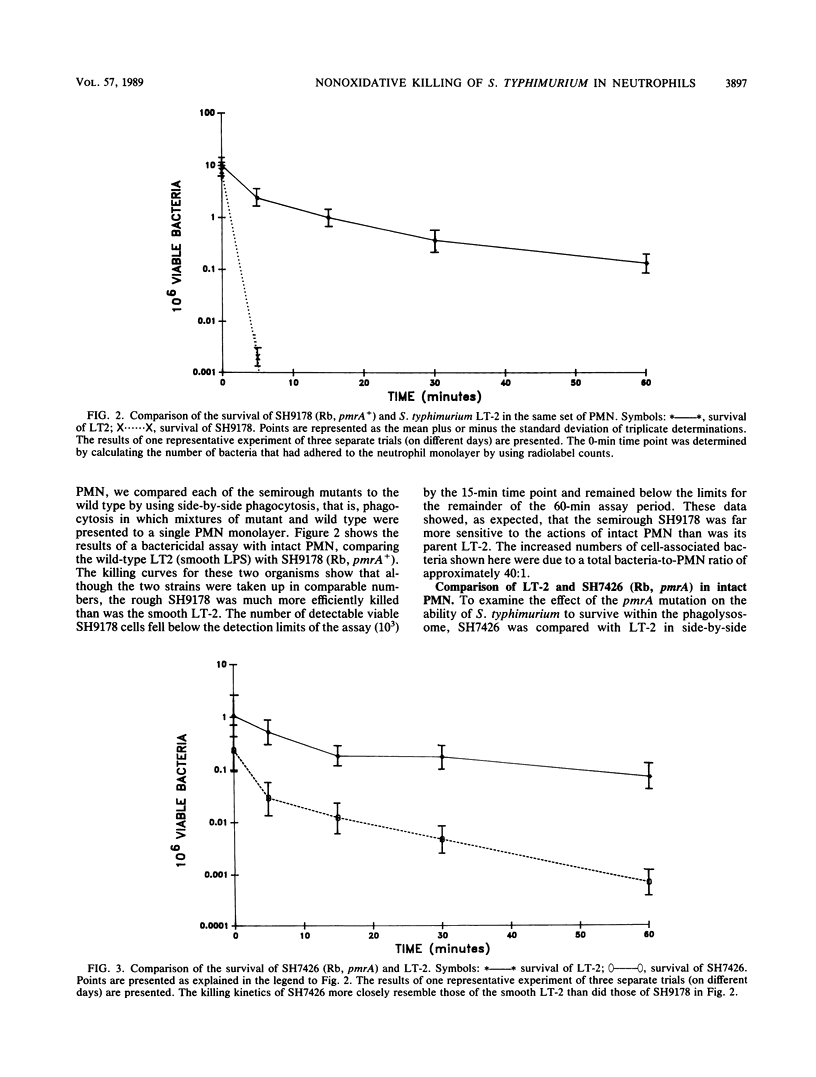
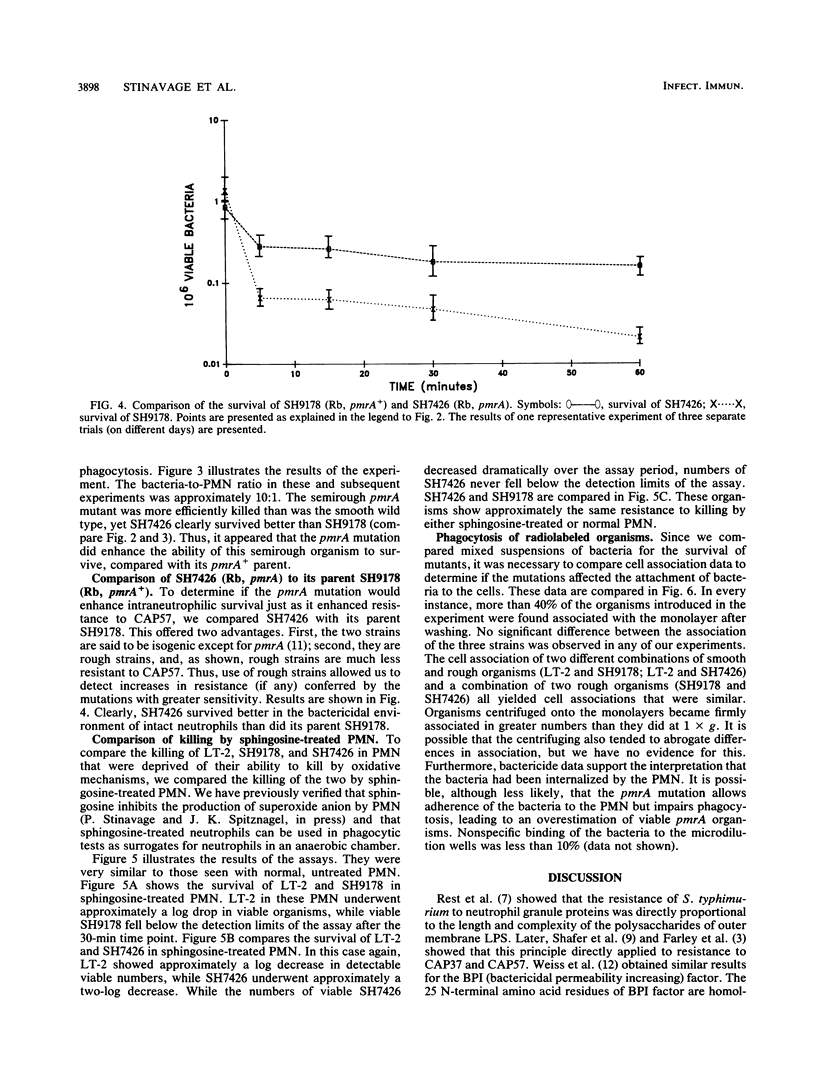
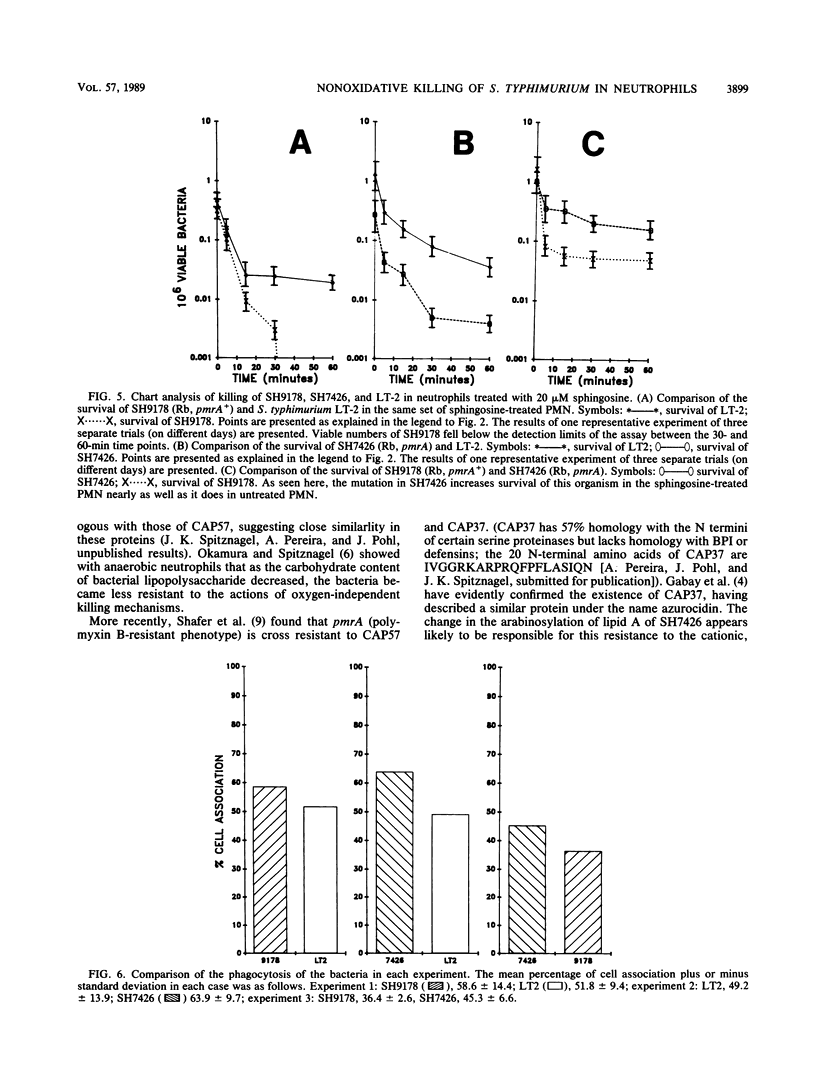
We have compared the intraleukocytic survival of isogenic strains of Salmonella typhimurium, whose outer membrane lipopolysaccharide differed in O antigen and lipid A composition and whose susceptibility to nonoxidative antimicrobial granule proteins of human polymorphonuclear neutrophilis (PMN) could be established. We found that the order of resistance to the bactericidal activity of intact PMN of the three bacterial strains utilized closely resembled their ordered resistance to the purified human cationic antimicrobial 57,000-dalton protein (CAP57). LT-2, a smooth wild-type strain, was far more resistant than SH9178, its rough (Rb LPS) mutant. It was most significant that SH7426, a polymyxin B-resistant pmrA mutant of SH9178, not only was substantially more resistant to CAP57 and to intraphagocytic killing than SH9178 but also came close to being as resistant as LT-2. These experiments confirm earlier work that showed the importance of the glycosyl groups of O antigens of S. typhimurium for their resistance to O2-independent antimicrobial phagocytosis by PMN. The surprising result was that a rough strain, very susceptible to bactericide, became substantially more resistant when a mutation led to its lipid A phosphoryl groups being 100% substituted with amino pentoses. Yet unresolved is whether the protection is due to the loss of negative charges on the lipid A, the substitution of sugar molecules in vulnerable loci in the outer membrane, or both.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey S. G., Shafer W. M., Spitznagel J. K. Anaerobiosis increases resistance of Neisseria gonorrhoeae to O2-independent antimicrobial proteins from human polymorphonuclear granulocytes. Infect Immun. 1985 Feb;47(2):401–407. doi: 10.1128/iai.47.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley M. M., Shafer W. M., Spitznagel J. K. Lipopolysaccharide structure determines ionic and hydrophobic binding of a cationic antimicrobial neutrophil granule protein. Infect Immun. 1988 Jun;56(6):1589–1592. doi: 10.1128/iai.56.6.1589-1592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Scott R. W., Campanelli D., Griffith J., Wilde C., Marra M. N., Seeger M., Nathan C. F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes: antagonism of granule cationic proteins by lipopolysaccharide. Infect Immun. 1979 Aug;25(2):597–602. doi: 10.1128/iai.25.2.597-602.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Spitznagel J. K. Outer membrane mutants of Salmonella typhimurium LT2 have lipopolysaccharide-dependent resistance to the bactericidal activity of anaerobic human neutrophils. Infect Immun. 1982 Jun;36(3):1086–1095. doi: 10.1128/iai.36.3.1086-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Myeloperoxidase-Cl--H2O2 bactericidal system: effect of bacterial membrane structure and growth conditions. Infect Immun. 1978 Mar;19(3):1110–1112. doi: 10.1128/iai.19.3.1110-1112.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Casey S. G., Spitznagel J. K. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophil granulocytes. Infect Immun. 1984 Mar;43(3):834–838. doi: 10.1128/iai.43.3.834-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid A mutants. J Bacteriol. 1981 Nov;148(2):426–434. doi: 10.1128/jb.148.2.426-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Muello K., Victor M., Elsbach P. The role of lipopolysaccharides in the action of the bactericidal/permeability-increasing neutrophil protein on the bacterial envelope. J Immunol. 1984 Jun;132(6):3109–3115. [PubMed] [Google Scholar]



