Abstract
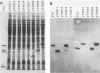
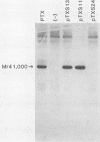
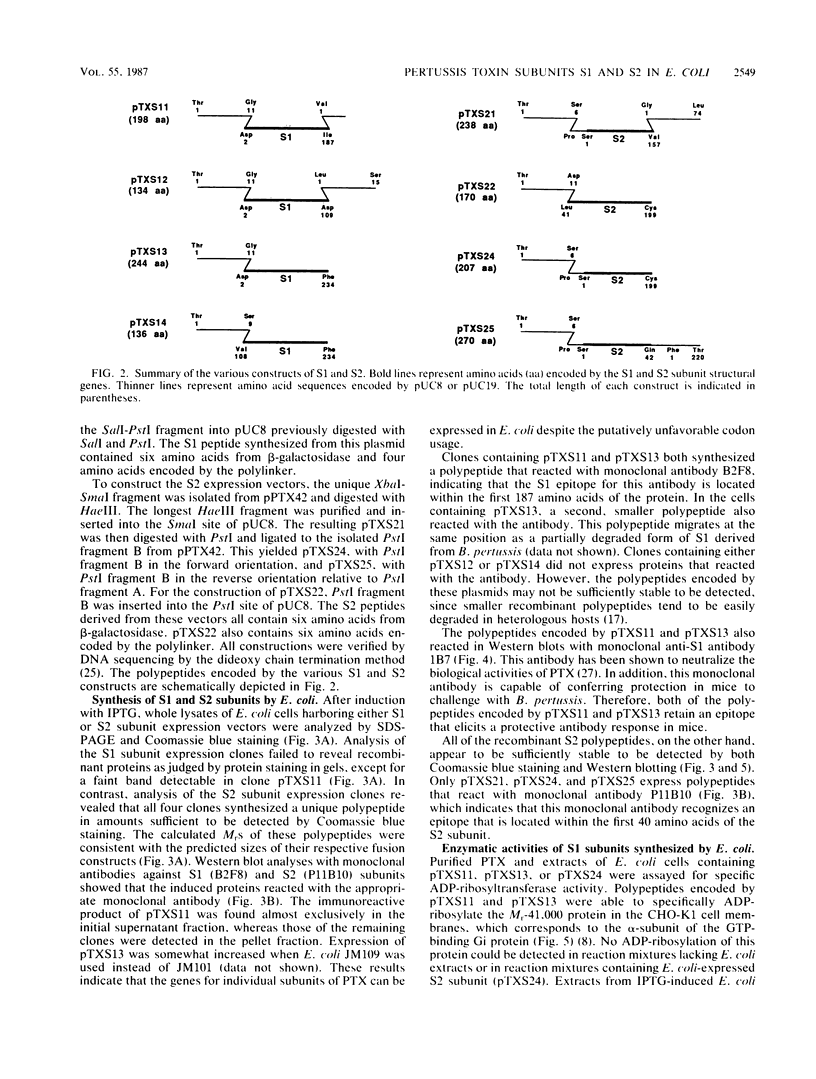
The genes encoding the S1 and S2 subunits of pertussis toxin were expressed in Escherichia coli under lac operon transcription and translation control with pUC8 and pUC18 as the expression vectors. Various versions of the subunits were detected with anti-S1 or anti-S2 monoclonal antibodies. Recombinant S1, but not S2, subunit contained the enzymatic NAD-glycohydrolase and NAD:Gi ADP-ribosyltransferase activities. Both activities were also expressed by a truncated version of the S1 subunit in which the 48 carboxy-terminal amino acid residues, including a predicted Rossman structure and one of the two cysteines, had been deleted. The epitope for an anti-S2 monoclonal antibody was localized to the N-terminal 40-amino-acid region of the S2 subunit. Both the S1 and S2 subunits expressed in E. coli reacted with human hyperimmune serum. The full length and the truncated recombinant S1 subunit also reacted in Western blots with a neutralizing and protective monoclonal anti-S1 antibody. The different versions of S1 and S2 subunits expressed in E. coli are useful for mapping active sites, epitopes, and regions that interact with receptors or the other subunits in the holotoxin. These recombinant subunits will also facilitate the development of a safer, new-generation vaccine against whooping cough.
Full text
PDF

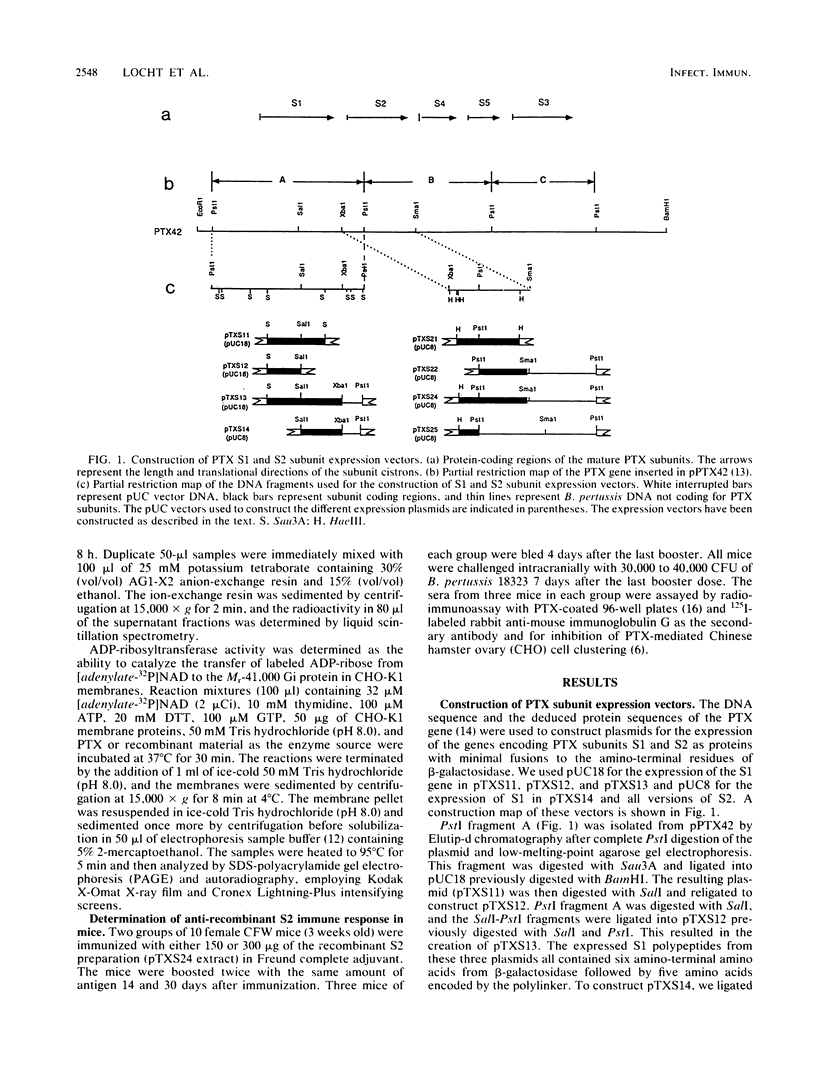



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Capiau C., Petre J., Van Damme J., Puype M., Vandekerckhove J. Protein-chemical analysis of pertussis toxin reveals homology between the subunits S2 and S3, between S1 and the A chains of enterotoxins of Vibrio cholerae and Escherichia coli and identifies S2 as the haptoglobin-binding subunit. FEBS Lett. 1986 Aug 18;204(2):336–340. doi: 10.1016/0014-5793(86)80839-0. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Lory S., Collier R. J. Ligand interactions of diphtheria toxin. III. Direct photochemical cross-linking of ATP and NAD to toxin. J Biol Chem. 1980 Dec 25;255(24):12020–12024. [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Interaction of monoclonal antibodies with pertussis toxin and its subunits. Infect Immun. 1984 Oct;46(1):195–201. doi: 10.1128/iai.46.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia J. A., Moss J., Hewlett E. L., Vaughan M. ADP-ribosylation of adenylate cyclase by pertussis toxin. Effects on inhibitory agonist binding. J Biol Chem. 1984 Jan 25;259(2):1086–1090. [PubMed] [Google Scholar]
- Katada T., Tamura M., Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983 Jul 1;224(1):290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locht C., Barstad P. A., Coligan J. E., Mayer L., Munoz J. J., Smith S. G., Keith J. M. Molecular cloning of pertussis toxin genes. Nucleic Acids Res. 1986 Apr 25;14(8):3251–3261. doi: 10.1093/nar/14.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Munoz J. J., Keith J. M. Detection of subunits of pertussis toxin in Tn5-induced Bordetella mutants deficient in toxin biological activity. Infect Immun. 1987 May;55(5):1309–1313. doi: 10.1128/iai.55.5.1309-1313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston F. A. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A., Burns D. L., Manclark C. R., Kaslow H. R., Hewlett E. L. Stimulation of the thiol-dependent ADP-ribosyltransferase and NAD glycohydrolase activities of Bordetella pertussis toxin by adenine nucleotides, phospholipids, and detergents. Biochemistry. 1986 May 6;25(9):2720–2725. doi: 10.1021/bi00357a066. [DOI] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Bernard C. C., Mackay I. R. Elicitation of experimental allergic encephalomyelitis (EAE) in mice with the aid of pertussigen. Cell Immunol. 1984 Jan;83(1):92–100. doi: 10.1016/0008-8749(84)90228-4. [DOI] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S., Judd R. C., Munoz J. J. Effect of proteolytic enzymes, storage and reduction on the structure and biological activity of pertussigen, a toxin from Bordetella pertussis. Dev Biol Stand. 1985;61:75–87. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Ito A., Chiba J., Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984 Nov;46(2):422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y., Ito A., Ohishi I. Effect of monoclonal antibody to pertussis toxin on toxin activity. Infect Immun. 1987 Apr;55(4):909–915. doi: 10.1128/iai.55.4.909-915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Wardlaw A. C., Parton R., Hooker M. J. Loss of protective antigen, histamine-sensitising factor and envelope polypeptides in cultural variants of Bordetella pertussis. J Med Microbiol. 1976 Feb;9(1):89–100. doi: 10.1099/00222615-9-1-89. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Burns D. L., Kanaho Y., Liu T. Y., Hewlett E. L., Moss J. ADP-ribosylation of transducin by pertussis toxin. J Biol Chem. 1985 Nov 5;260(25):13478–13482. [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]