Abstract
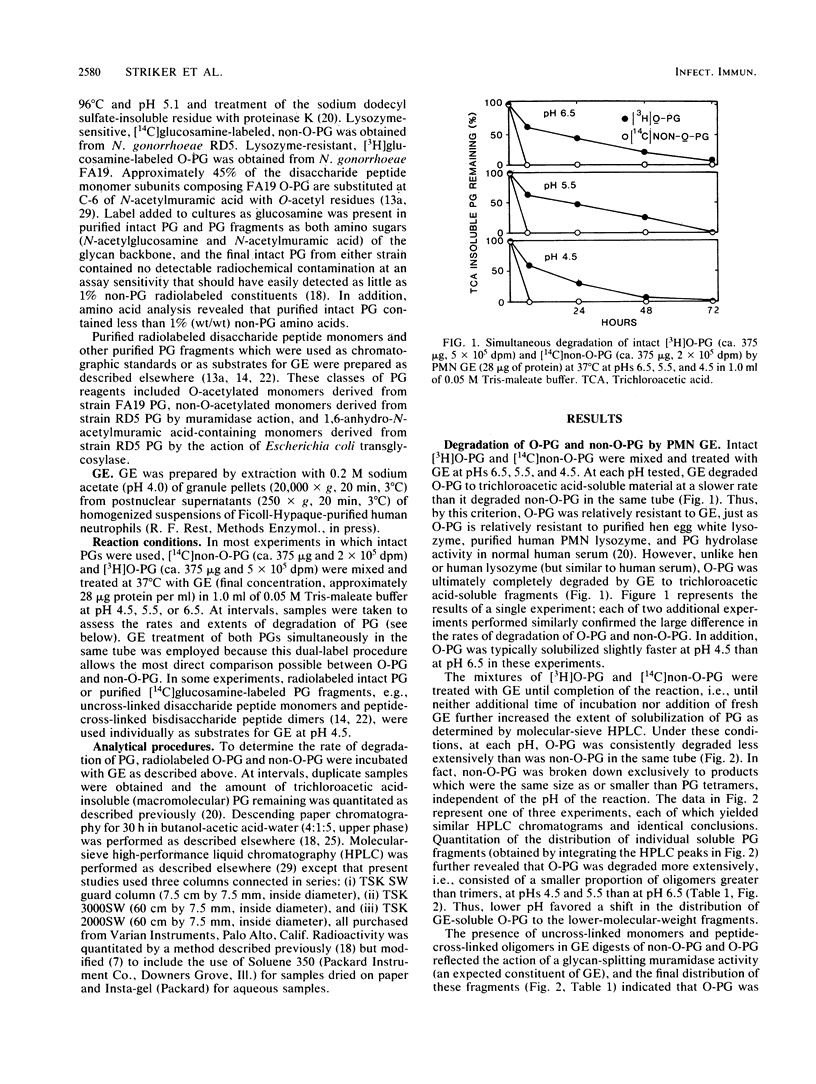
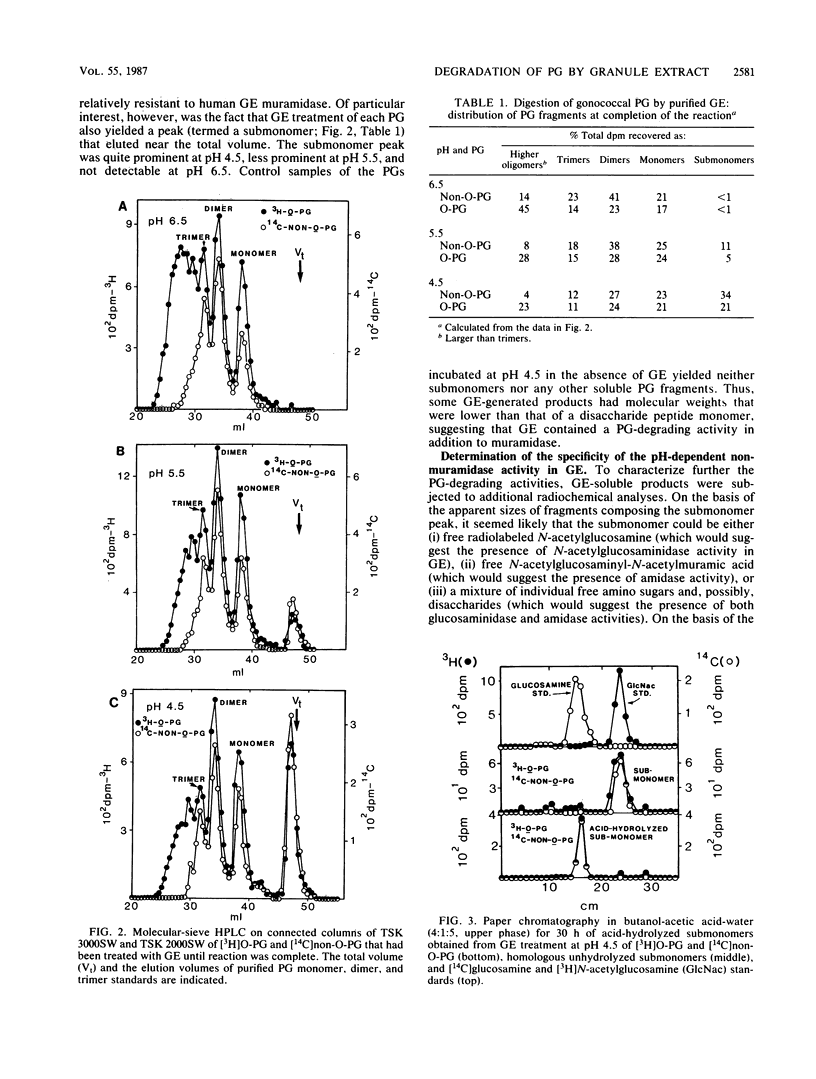
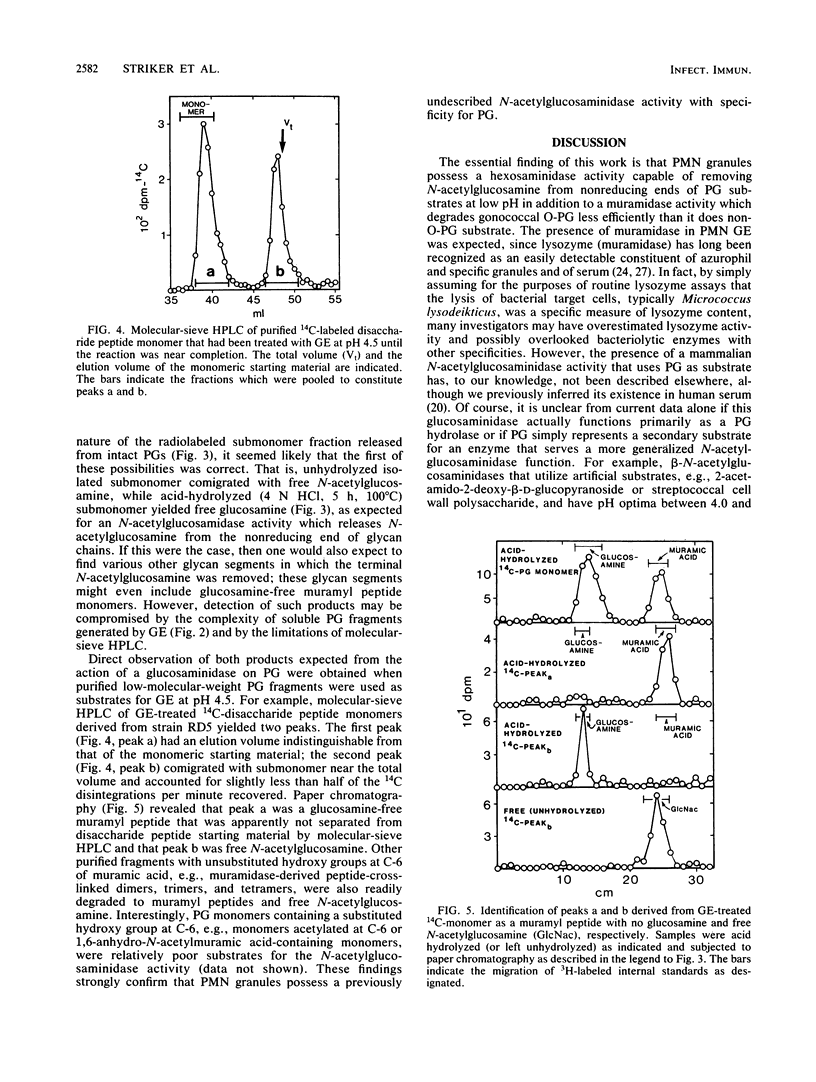
The degradation of purified Neisseria gonorrhoeae peptidoglycan (PG) by granule extract derived from normal human polymorphonuclear leukocytes was examined. Hen egg lysozyme-resistant, extensively O-acetylated [3H]PG (O-PG) from strain FA19 and lysozyme-sensitive, non-O-acetylated [14C]PG (non-O-PG) from strain RD5 (each containing label in both glucosamine and muramic acid) were mixed and incubated with granule extract at pHs 4.5, 5.5, and 6.5. The rate of degradation of O-PG was uniformly slower than that of non-O-PG in the same tube, but ultimately, even the O-PG was rendered completely soluble. Molecular-sieve high-performance liquid chromatography revealed that both PGs were degraded by granule extract at the pH values tested to disaccharide peptide monomers and peptide-cross-linked oligomers, reflecting the action of human lysozyme. Of particular interest was the appearance of a peak containing free N-acetylglucosamine which was quite prominent in reaction mixtures at pH 4.5, less prominent at pH 5.5, and not detectable at pH 6.5. Free N-acetylglucosamine was not released from control PG samples at any pH in the absence of granule extract. Treatment of purified gonococcal PG monomers with granule extract at pH 4.5 yielded exclusively free N-acetylglucosamine and muramyl peptides with no N-acetylglucosamine. These data suggest that granule extract contains a previously undescribed pH-dependent N-acetylglucosaminidase with specificity for PG as well as an N-acetylmuramidase activity that degrades O-PG less efficiently than it does non-O-PG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub E. M., McCarty M. Intraphagocytic beta-N-acetylglucosaminidase. Properties of the enzyme and its activity on group A streptococcal carbohydrate in comparison with a soil bacillus enzyme. J Exp Med. 1968 Apr 1;127(4):833–851. doi: 10.1084/jem.127.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P., Revilla M. G., Cabezas J. A. Studies on blood serum beta-N-acetylglucosaminidases from several mammalian species--separation of different enzyme forms. Comp Biochem Physiol B. 1978;61(4):581–585. doi: 10.1016/0305-0491(78)90053-6. [DOI] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T. J., Wallsmith D. E., Rosenthal R. S. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect Immun. 1986 May;52(2):600–608. doi: 10.1128/iai.52.2.600-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkening W. J., Nogami W., Martin S. A., Rosenthal R. S. Structure of Bordetella pertussis peptidoglycan. J Bacteriol. 1987 Sep;169(9):4223–4227. doi: 10.1128/jb.169.9.4223-4227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. M., Davenne D., Walter J., Shoham S., Kubillus S. L., Rosenthal R. S., Martin S. A., Biemann K. Bacterial peptidoglycans as modulators of sleep. II. Effects of muramyl peptides on the structure of rabbit sleep. Brain Res. 1987 Feb 17;403(2):258–266. doi: 10.1016/0006-8993(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Rosenthal R. S., Martin S. A., Walter J., Davenne D., Shoham S., Kubillus S. L., Biemann K. Bacterial peptidoglycans as modulators of sleep. I. Anhydro forms of muramyl peptides enhance somnogenic potency. Brain Res. 1987 Feb 17;403(2):249–257. doi: 10.1016/0006-8993(87)90062-x. [DOI] [PubMed] [Google Scholar]
- Ladesić B., Tomasić J., Kveder S., Hrsak I. The metabolic fate of 14C-labeled immunoadjuvant peptidoglycan monomer. II. In vitro studies. Biochim Biophys Acta. 1981 Nov 18;678(1):12–17. doi: 10.1016/0304-4165(81)90042-8. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Rosenthal R. S., Biemann K. Fast atom bombardment mass spectrometry and tandem mass spectrometry of biologically active peptidoglycan monomers from Neisseria gonorrhoeae. J Biol Chem. 1987 Jun 5;262(16):7514–7522. [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Petersen B. H., Rosenthal R. S. Complement consumption gonococcal peptidoglycan. Infect Immun. 1982 Feb;35(2):442–448. doi: 10.1128/iai.35.2.442-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Subcellular distribution of glycosidases in human polymorphonuclear leucocytes. Biochem J. 1978 Jul 15;174(1):53–59. doi: 10.1042/bj1740053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Blundell J. K., Perkins H. R. Strain-related differences in lysozyme sensitivity and extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1982 Aug;37(2):826–829. doi: 10.1128/iai.37.2.826-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Folkening W. J., Miller D. R., Swim S. C. Resistance of O-acetylated gonococcal peptidoglycan to human peptidoglycan-degrading enzymes. Infect Immun. 1983 Jun;40(3):903–911. doi: 10.1128/iai.40.3.903-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Martinez R. J. Lysozyme: primary bactericidin in human plasma serum active against Bacillus subtilis. Infect Immun. 1978 Jun;20(3):782–791. doi: 10.1128/iai.20.3.782-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Effect of penicillin G on release of peptidoglycan fragments by Neisseria gonorrhoeae: characterization of extracellular products. Antimicrob Agents Chemother. 1981 Jul;20(1):98–103. doi: 10.1128/aac.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Stimpson S. A., Lerch R. A., Cleland D. R., Yarnall D. P., Clark R. L., Cromartie W. J., Schwab J. H. Effect of acetylation on arthropathic activity of group A streptococcal peptidoglycan-polysaccharide fragments. Infect Immun. 1987 Jan;55(1):16–23. doi: 10.1128/iai.55.1.16-23.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swim S. C., Gfell M. A., Wilde C. E., 3rd, Rosenthal R. S. Strain distribution in extents of lysozyme resistance and O-acetylation of gonococcal peptidoglycan determined by high-performance liquid chromatography. Infect Immun. 1983 Nov;42(2):446–452. doi: 10.1128/iai.42.2.446-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valinger Z., Ladesić B., Tomasić J. Partial purification and characterization of N-acetylmuramyl-L-alanine amidase from human and mouse serum. Biochim Biophys Acta. 1982 Feb 4;701(1):63–71. doi: 10.1016/0167-4838(82)90313-2. [DOI] [PubMed] [Google Scholar]



