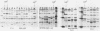Abstract
Levels of genotypic and phenotypic diversity among 23 ampicillin-resistant, non-beta-lactamase-producing (Ampr NBLP) isolates of serologically nontypeable Haemophilus influenzae recovered from the respiratory tract were determined by multilocus enzyme electrophoresis, auxotroph testing in chemically defined media, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of penicillin-binding proteins (PBPs). Twenty distinctive multilocus enzyme genotypes were identified, among which the average level of genetic diversity per locus was equivalent to that in the species as a whole. Hence, a single, recent origin for Ampr NBLP strains is excluded. Of the growth factors tested, a requirement for methionine was significantly associated with the Ampr NBLP phenotype. In contrast to the relative homogeneity of the PBP profiles of the ampicillin-susceptible strains tested (8 PBPs detected), the PBP profiles of the Ampr NBLP strains exhibited marked heterogeneity (5 to 10 PBPs detected). Care should be taken in interpreting changes in PBP profiles and in associating these profiles with resistance for species such as H. influenzae that demonstrate variability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Makover S. D., Wright R., Telep E. Penicillin-binding proteins in Haemophilus influenzae. Antimicrob Agents Chemother. 1981 Apr;19(4):584–588. doi: 10.1128/aac.19.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Clausen C., Stull T. L., Needham C., Williams J. D., Smith A. L. Failure to detect ampicillin-resistant, non-beta-lactamase-producing Haemophilus influenzae by standard disk susceptibility testing. Antimicrob Agents Chemother. 1986 Aug;30(2):274–280. doi: 10.1128/aac.30.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Syriopoulou V. P., Gandy S. L., Ward J. I., Smith A. L. Molecular epidemiology of plasmid-mediated ampicillin resistance in Haemophilus influenzae type b isolates from Alaska. J Infect Dis. 1985 Jun;151(6):1061–1072. doi: 10.1093/infdis/151.6.1061. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Barenkamp S. J., Granoff D. M., Selander R. K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986 Apr;52(1):183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Granoff D. M., Pattison P. E., Selander R. K. A population genetic framework for the study of invasive diseases caused by serotype b strains of Haemophilus influenzae. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5078–5082. doi: 10.1073/pnas.82.15.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Mechanism of resistance of an ampicillin-resistant, beta-lactamase-negative clinical isolate of Haemophilus influenzae type b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott-Howard J., Williams J. D. Increase in antibiotic resistance in Haemophilus influenzae in the United Kingdom since 1977: report of study group. Br Med J (Clin Res Ed) 1982 May 29;284(6329):1597–1599. doi: 10.1136/bmj.284.6329.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfass D. A., Mendelman P. M., Chaffin D. O., Needham C. A. Ampicillin resistance and penicillin-binding proteins of Haemophilus influenzae. J Gen Microbiol. 1986 Oct;132(10):2855–2861. doi: 10.1099/00221287-132-10-2855. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Biochemical and genetical approaches to the mechanism of action of penicillin. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):273–283. doi: 10.1098/rstb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Tebbutt G. M. A chemotyping scheme for clinical isolates of Haemophilus influenzae. J Med Microbiol. 1984 Jun;17(3):335–345. doi: 10.1099/00222615-17-3-335. [DOI] [PubMed] [Google Scholar]