Abstract
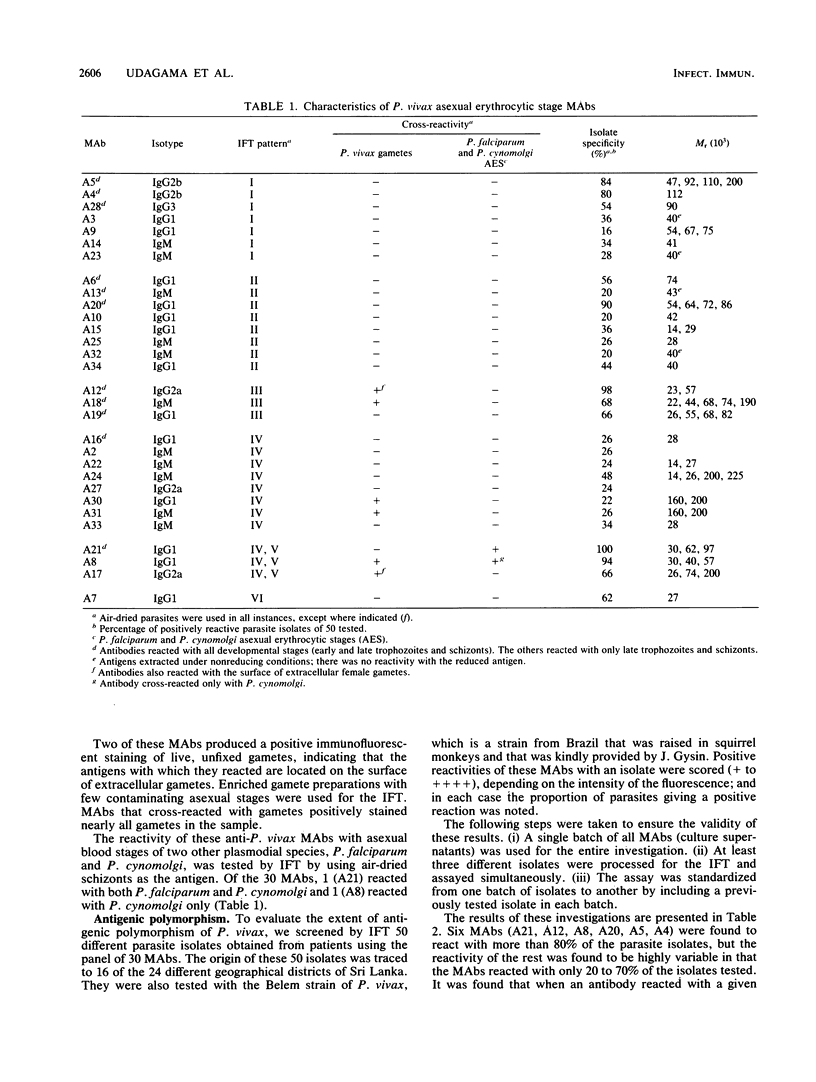
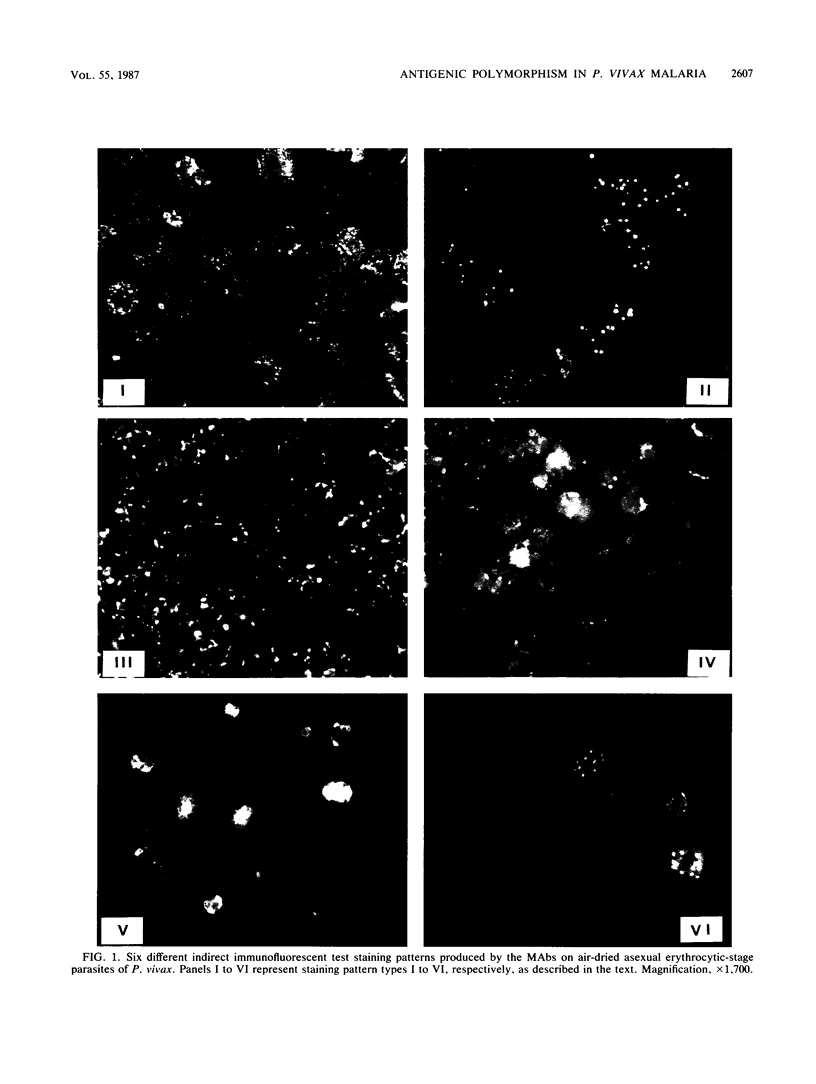
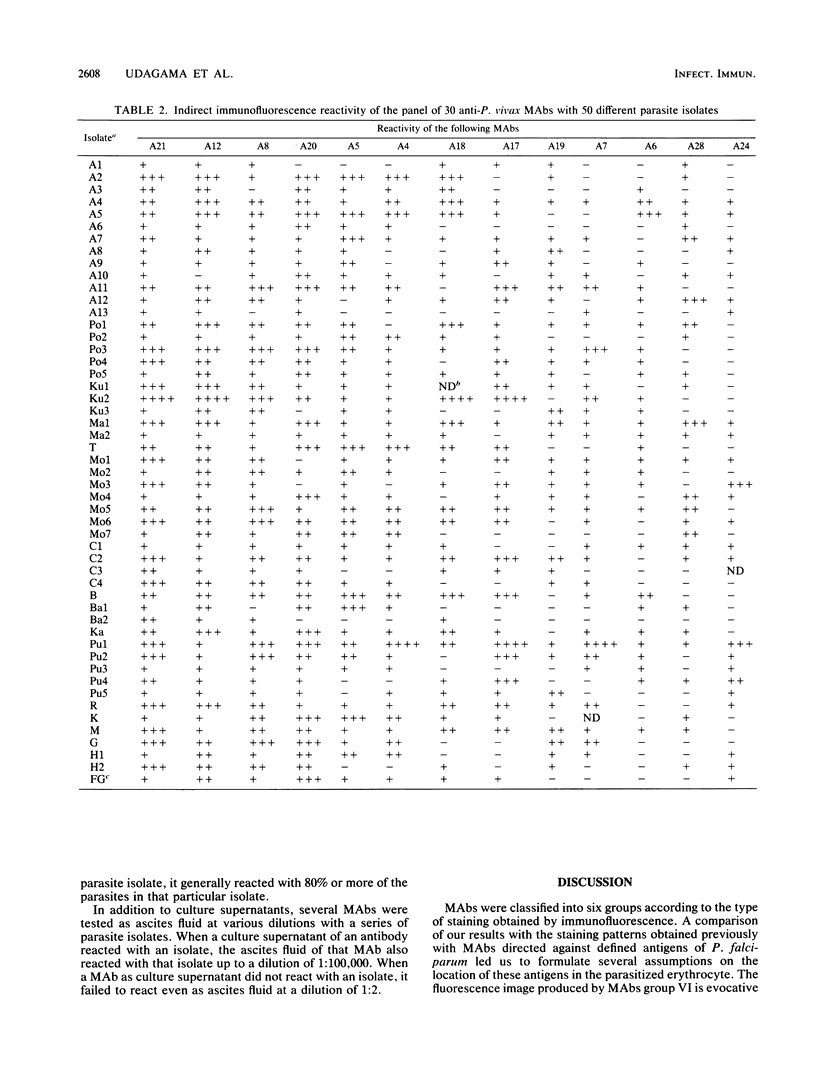
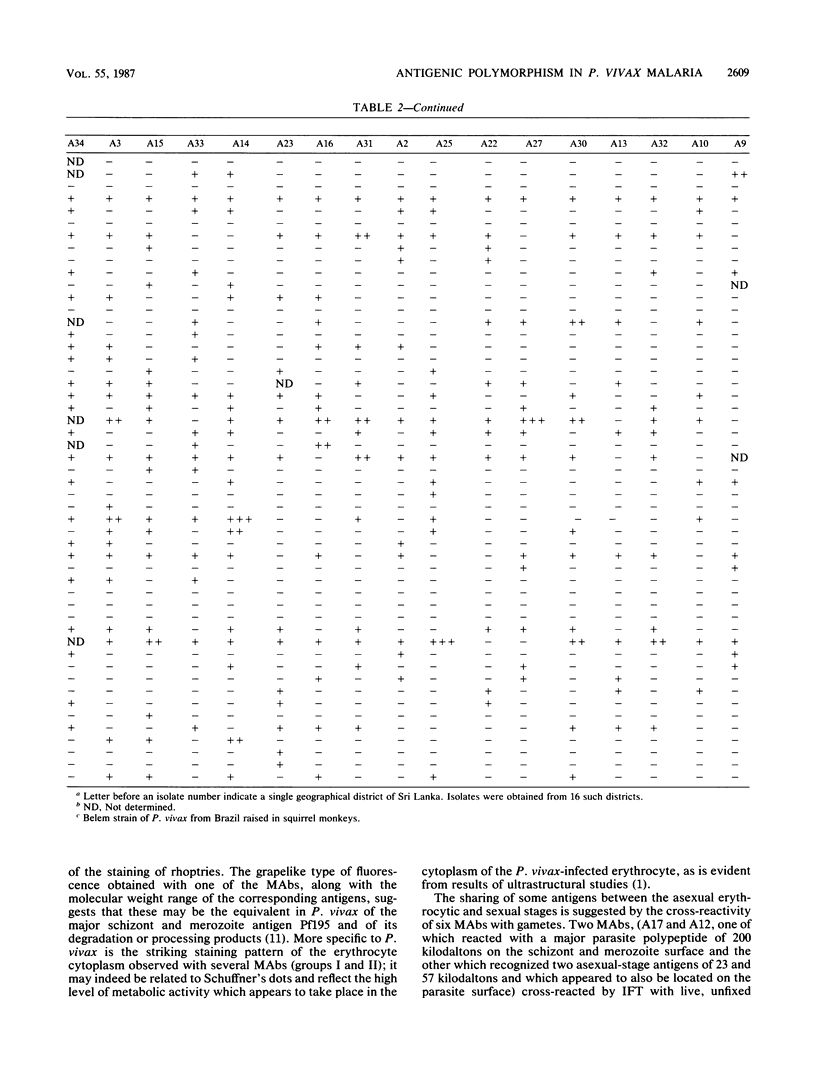
A panel of 30 monoclonal antibodies was established against asexual erythrocytic stages of Plasmodium vivax and used to investigate the antigenic composition of the parasite. At least 38 different antigenic polypeptides of P. vivax were characterized by the Western blot technique. The possible location of these antigens, as well as their stage and species specificity, was determined on the basis of the staining patterns produced by these antibodies on air-dried parasites in the indirect immunofluorescence test. Immunofluorescence performed with 30 different monoclonal antibodies on 50 different isolates of P. vivax obtained from patients showed that a high level of antigenic polymorphism prevailed in P. vivax. Only six monoclonal antibodies reacted with epitopes that were represented in more than 80% of parasite isolates, and therefore, appeared to be relatively conserved among different isolates. The other 24 monoclonal antibodies reacted with only 20 to 70% of parasite isolates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Miller L. H., Rabbege J. Caveola--vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P cynomolgi. Unique structures related to Schüffner's dots. Am J Pathol. 1975 May;79(2):285–300. [PMC free article] [PubMed] [Google Scholar]
- Arnot D. E., Barnwell J. W., Tam J. P., Nussenzweig V., Nussenzweig R. S., Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985 Nov 15;230(4727):815–818. doi: 10.1126/science.2414847. [DOI] [PubMed] [Google Scholar]
- Barnwell J. W., Howard R. J., Coon H. G., Miller L. H. Splenic requirement for antigenic variation and expression of the variant antigen on the erythrocyte membrane in cloned Plasmodium knowlesi malaria. Infect Immun. 1983 Jun;40(3):985–994. doi: 10.1128/iai.40.3.985-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Gwadz R. W., Green I. Plasmodium gallinaceum: Transmission-blocking immunity in chickens. II. The effect of antigamete antibodies in vitro and in vivo and their elaboration during infection. Exp Parasitol. 1979 Apr;47(2):194–208. doi: 10.1016/0014-4894(79)90073-0. [DOI] [PubMed] [Google Scholar]
- David P. H., Hudson D. E., Hadley T. J., Klotz F. W., Miller L. H. Immunization of monkeys with a 140 kilodalton merozoite surface protein of Plasmodium knowlesi malaria: appearance of alternate forms of this protein. J Immunol. 1985 Jun;134(6):4146–4152. [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Burkot T. R., Rener J., Kaushal D. C., Williams J. L. Effects of transmission-blocking monoclonal antibodies on different isolates of Plasmodium falciparum. Infect Immun. 1985 Jun;48(3):611–616. doi: 10.1128/iai.48.3.611-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz R. W., Cochrane A. H., Nussenzweig V., Nussenzweig R. S. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ. 1979;57 (Suppl 1):165–173. [PMC free article] [PubMed] [Google Scholar]
- Hall R., McBride J., Morgan G., Tait A., Zolg J. W., Walliker D., Scaife J. Antigens of the erythrocytes stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol Biochem Parasitol. 1983 Mar;7(3):247–265. doi: 10.1016/0166-6851(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Handunnetti S. M., Mendis K. N., David P. H. Antigenic variation of cloned Plasmodium fragile in its natural host Macaca sinica. Sequential appearance of successive variant antigenic types. J Exp Med. 1987 May 1;165(5):1269–1283. doi: 10.1084/jem.165.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Uni S., Aikawa M. Isolation of a Plasmodium falciparum rhoptry protein. Mol Biochem Parasitol. 1985 Mar;14(3):293–303. doi: 10.1016/0166-6851(85)90057-x. [DOI] [PubMed] [Google Scholar]
- Homewood C. A., Neame K. D. A comparison of methods used for the removal of white cells from malaria-infected blood. Ann Trop Med Parasitol. 1976 Jun;70(2):249–251. doi: 10.1080/00034983.1976.11687119. [DOI] [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983 Apr 1;157(4):1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihalamulla R. L., Mendis K. N. Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1987;81(1):25–28. doi: 10.1016/0035-9203(87)90271-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Miller L. H., Howard R. J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984 Jun 1;159(6):1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Walliker D., Morgan G. Antigenic diversity in the human malaria parasite Plasmodium falciparum. Science. 1982 Jul 16;217(4556):254–257. doi: 10.1126/science.6178159. [DOI] [PubMed] [Google Scholar]
- McBride J. S., Welsby P. D., Walliker D. Serotyping Plasmodium falciparum from acute human infections using monoclonal antibodies. Trans R Soc Trop Med Hyg. 1984;78(1):32–34. doi: 10.1016/0035-9203(84)90167-6. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Lal A. A., de la Cruz V. F., Miller L. H., Maloy W. L., Charoenvit Y., Beaudoin R. L., Guerry P., Wistar R., Jr, Hoffman S. L. Sequence of the immunodominant epitope for the surface protein on sporozoites of Plasmodium vivax. Science. 1985 Dec 20;230(4732):1381–1383. doi: 10.1126/science.2416057. [DOI] [PubMed] [Google Scholar]
- Mitchell G. H., Richards W. H., Butcher G. A., Cohen S. Merozoite vaccination of douroucouli monkeys against falciparum malaria. Lancet. 1977 Jun 25;1(8026):1335–1338. doi: 10.1016/s0140-6736(77)92551-x. [DOI] [PubMed] [Google Scholar]
- Munesinghe Y. D., Mendis K. N., Carter R. Anti-gamete antibodies block transmission of human vivax malaria to mosquitoes. Parasite Immunol. 1986 May;8(3):231–238. doi: 10.1111/j.1365-3024.1986.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Saul A., Myler P., Elliott T., Kidson C. Purification of mature schizonts of Plasmodium falciparum on colloidal silica gradients. Bull World Health Organ. 1982;60(5):755–759. [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Bushell G. R., Cooper J. A., Saul A. J., Upcroft J. A., Kidson C. A rhoptry antigen of Plasmodium falciparum contains conserved and variable epitopes recognized by inhibitory monoclonal antibodies. Mol Biochem Parasitol. 1986 Feb;18(2):183–195. doi: 10.1016/0166-6851(86)90037-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Graves P. M., Carter R., Aikawa M., Miller L. H. Plasmodium falciparum: effect of time in continuous culture on binding to human endothelial cells and amelanotic melanoma cells. Exp Parasitol. 1983 Oct;56(2):207–214. doi: 10.1016/0014-4894(83)90064-4. [DOI] [PubMed] [Google Scholar]
- Voller A., O'Neill P. Immunofluorescence method suitable for large-scale application to malaria. Bull World Health Organ. 1971;45(4):524–529. [PMC free article] [PubMed] [Google Scholar]
- Wilson R. J. Serotyping Plasmodium falciparum malaria with S-antigens. Nature. 1980 Apr 3;284(5755):451–452. doi: 10.1038/284451a0. [DOI] [PubMed] [Google Scholar]




