Abstract
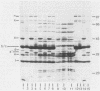
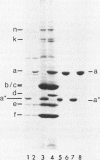
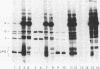
Protein a (46,000 molecular weight [46K]) was purified from outer membranes of Haemophilus influenzae type b by a relatively simple procedure. Spontaneously shed outer membranes from a 24-h, 12-liter culture of an unencapsulated variant of strain Eag were combined with outer membranes released from the cells by Tris buffer and extracted with the nonionic detergent octylpolyoxyethylene. The extract was then subjected to open column chromatography on Sephacryl S-200 and Trisacryl-carboxymethyl to yield 7.5 mg of protein a from 180 mg of outer membrane protein. Approximately 99% of the protein in this preparation was protein a; in addition, the preparation contained 1.25% (wt/wt) lipopolysaccharide and had a residual detergent/protein ratio of 1.6:1 (wt/wt). Antibodies to the preparation were induced in rabbits by using alum as an adjuvant. As determined by immunoblotting, the great preponderance of antibodies induced were specific for protein a. However, very low levels of antibodies to several other outer membrane components, which were not apparent on gels of the pure preparation of protein a, were also induced. Preimmune and postimmune sera, after depletion of antibodies to capsular polysaccharide and lipopolysaccharide, were tested for biological activity against H. influenzae type b. Compared with preimmune serum, postimmune serum was bactericidal in vitro against strain Eag (the only strain tested) and offered significant protection (P less than 0.01) to infant rats against infection by all four strains tested, two of which had a protein a that was larger (47K) than the 46K protein a in the preparation. These results indicate that protein a should be considered as a vaccine to prevent H. influenzae type b disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. Intrinsic tritium labeling of the capsular polysaccharide antigen of Haemophilus influenzae type B. J Immunol. 1978 Mar;120(3):866–870. [PubMed] [Google Scholar]
- Chen Y. H., Hancock R. E., Mishell R. I. Mitogenic effects of purified outer membrane proteins from Pseudomonas aeruginosa. Infect Immun. 1980 Apr;28(1):178–184. doi: 10.1128/iai.28.1.178-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K. M., Decker M. D., Palmer P., Porch C. R., Sell S. H., Wright P. F. Lack of comparability between commonly used serological assays of immune response to Haemophilus influenzae vaccine. J Infect Dis. 1987 Feb;155(2):283–291. doi: 10.1093/infdis/155.2.283. [DOI] [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Anderson P., Loeb M. R., Smith D. H. A polysaccharide-protein complex from Haemophilus influenzae type b. II. Human antibodies to its somatic components. J Infect Dis. 1981 Dec;144(6):521–529. doi: 10.1093/infdis/144.6.521. [DOI] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb M. R. Immunoblot method for identifying surface components, determining their cross-reactivity, and investigating cell topology: results with Haemophilus influenzae type b. Anal Biochem. 1984 Nov 15;143(1):196–204. doi: 10.1016/0003-2697(84)90576-1. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Deich R. A., Connelly C. Cloning of chromosomal DNA from Haemophilus influenzae. Its use for studying the expression of type b capsule and virulence. J Clin Invest. 1984 Feb;73(2):298–306. doi: 10.1172/JCI111214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. T., EITZMAN D. V. THE DEVELOPMENT OF THE IMMUNE RESPONSE. CHARACTERIZATION OF THE RESPONSE OF THE HUMAN INFANT AND ADULT TO IMMUNIZATION WITH SALMONELLA VACCINES. Pediatrics. 1964 Feb;33:163–183. [PubMed] [Google Scholar]
- Schreiber J. R., Barrus V., Cates K. L., Siber G. R. Functional characterization of human IgG, IgM, and IgA antibody directed to the capsule of Haemophilus influenzae type b. J Infect Dis. 1986 Jan;153(1):8–16. doi: 10.1093/infdis/153.1.8. [DOI] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Frasch C. E. Development of a Neisseria meningitidis group B serotype 2b protein vaccine and evaluation in a mouse model. Infect Immun. 1984 Nov;46(2):408–414. doi: 10.1128/iai.46.2.408-414.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Anderson P., Smith D. H. The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis. 1977 Jan;135(1):34–41. doi: 10.1093/infdis/135.1.34. [DOI] [PubMed] [Google Scholar]