Abstract
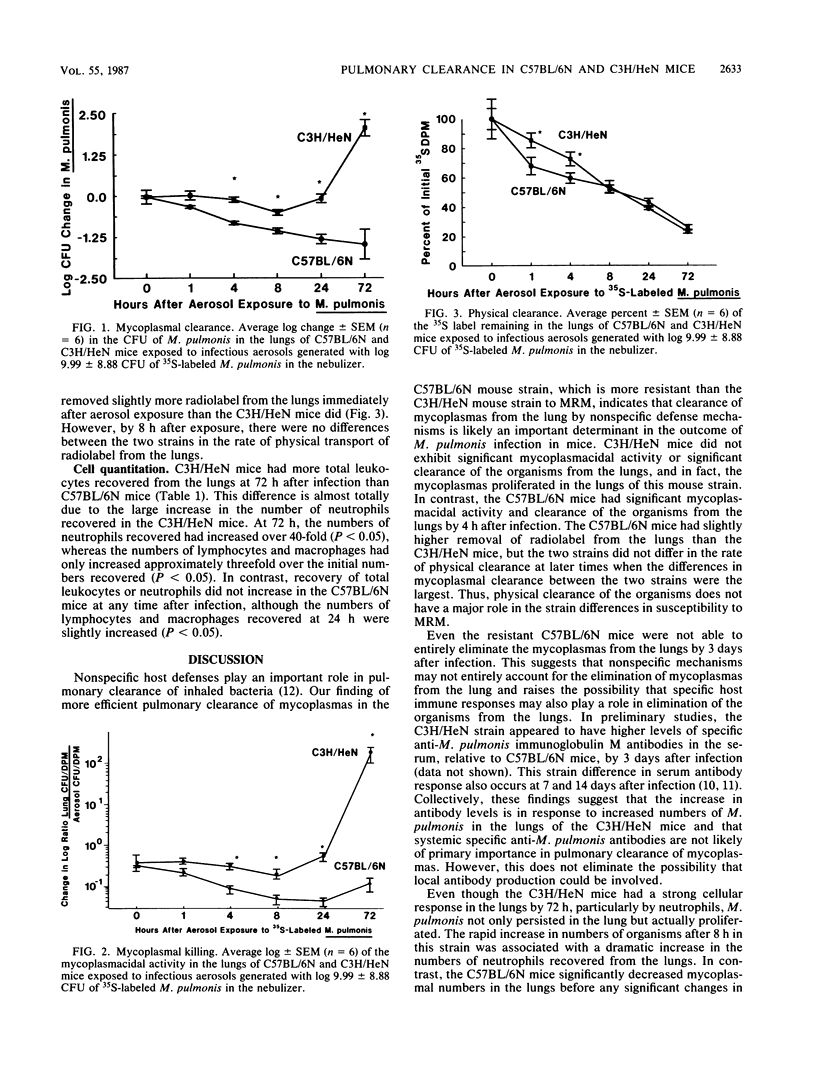
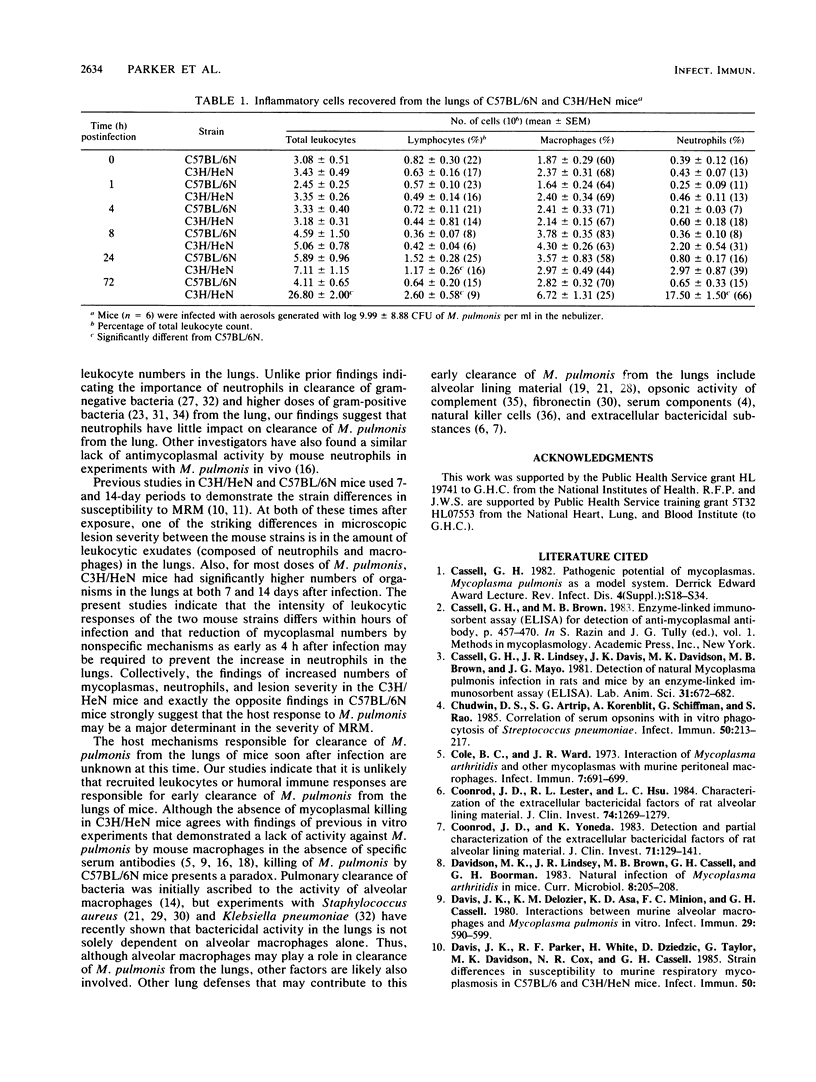
In C57BL/6N and C3H/HeN mice known to be free of all murine pathogens and matched for age, sex, and environmental factors, pulmonary clearance was measured over a 72-h time period after exposure to infectious aerosols of 35S-labeled Mycoplasma pulmonis. Reduced clearance of M. pulmonis in C3H/HeN mice relative to C57BL/6N mice was primarily due to impaired mycoplasmacidal activity in the lungs of the C3H/HeN mice. The C3H/HeN mice also had a slightly slower rate of mechanical transport of radiolabel from the lungs in the first 4 h after infection relative to the C57BL/6N mice but not at any later times. By 72 h after infection (relative to 0 h, C3H/HeN mice had an over 4,000% (1.75 X 10(7) versus 4.30 X 10(5] increase in neutrophils and an over 18,000% (more than 2 orders of magnitude) increase in numbers of M. pulmonis recovered from mechanically disaggregated lungs. In contrast, C57BL/6N mice reduced the number of M. pulmonis present by over 83% (nearly 2 orders of magnitude) before any increase in inflammatory cells, which was only a slight increase in lymphocytes and macrophages at 24 h after infection. These results directly link decreased mycoplasmal pulmonary clearance in C3H/HeN mice with the increased susceptibility to, and severity of, murine respiratory mycoplasmosis observed in this strain. The resistance of C57BL/6N mice appears to be related to nonspecific host defense mechanisms responsible for limiting the extent of infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassell G. H. Derrick Edward Award Lecture. The pathogenic potential of mycoplasmas: Mycoplasma pulmonis as a model. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S18–S34. doi: 10.1093/clinids/4.supplement_1.s18. [DOI] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K., Davidson M. K., Brown M. B., Mayo J. G. Detection of natural Mycoplasma pulmonis infection in rats and mice by an enzyme linked immunosorbent assay (ELISA). Lab Anim Sci. 1981 Dec;31(6):676–682. [PubMed] [Google Scholar]
- Chudwin D. S., Artrip S. G., Korenblit A., Schiffman G., Rao S. Correlation of serum opsonins with in vitro phagocytosis of Streptococcus pneumoniae. Infect Immun. 1985 Oct;50(1):213–217. doi: 10.1128/iai.50.1.213-217.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Lester R. L., Hsu L. C. Characterization of the extracellular bactericidal factors of rat alveolar lining material. J Clin Invest. 1984 Oct;74(4):1269–1279. doi: 10.1172/JCI111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Yoneda K. Detection and partial characterization of antibacterial factor(s) in alveolar lining material of rats. J Clin Invest. 1983 Jan;71(1):129–141. doi: 10.1172/JCI110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Delozier K. M., Asa D. K., Minion F. C., Cassell G. H. Interactions between murine alveolar macrophages and Mycoplasma pulmonis in vitro. Infect Immun. 1980 Aug;29(2):590–599. doi: 10.1128/iai.29.2.590-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Parker R. F., White H., Dziedzic D., D'Arcy J., Cassell G. H. Development of an aerosol model of murine respiratory mycoplasmosis in mice. Infect Immun. 1986 Oct;54(1):194–201. doi: 10.1128/iai.54.1.194-201.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. FACTORS INFLUENCING THE CLEARANCE OF BACTERIA BY THE LUNG. J Clin Invest. 1964 Apr;43:769–776. doi: 10.1172/JCI104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Goldstein E. A method for quantitating intrapulmonary bacterial inactivation in individual animals. J Lab Clin Med. 1966 Oct;68(4):669–677. [PubMed] [Google Scholar]
- Green G. M. Similarities of host defense mechanisms against pulmonary infectious diseases in animals and man. J Toxicol Environ Health. 1984;13(2-3):471–478. doi: 10.1080/15287398409530510. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Taylor G. Interaction of mycoplasmas and phagocytes. Yale J Biol Med. 1983 Sep-Dec;56(5-6):643–648. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction in vitro of Mycoplasma pulmonis with mouse peritoneal macrophages and L-cells. J Exp Med. 1971 Feb 1;133(2):231–259. doi: 10.1084/jem.133.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Lindsey J. R., Cassell H. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Models for studying mycoplasmosis of the respiratory tract. Am J Pathol. 1973 Jul;72(1):63–90. [PMC free article] [PubMed] [Google Scholar]
- Lipscomb M. F., Onofrio J. M., Nash E. J., Pierce A. K., Toews G. B. A morphological study of the role of phagocytes in the clearance of Staphylococcus aureus from the lung. J Reticuloendothel Soc. 1983 Jun;33(6):429–442. [PubMed] [Google Scholar]
- Minion F. C., Cassell G. H., Pnini S., Kahane I. Multiphasic interactions of Mycoplasma pulmonis with erythrocytes defined by adherence and hemagglutination. Infect Immun. 1984 May;44(2):394–400. doi: 10.1128/iai.44.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Cox C. D., Pesanti E. L. Pseudomonas aeruginosa clearance in mice: comparison of tissue, strain, and corticosteroid effects. Infect Immun. 1984 Mar;43(3):901–905. doi: 10.1128/iai.43.3.901-905.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Fick R. B., Jr Candidacidal factors in murine bronchoalveolar lavage fluid. Infect Immun. 1987 Mar;55(3):541–546. doi: 10.1128/iai.55.3.541-546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Pesanti E. L. Nonphagocytic clearance of Staphylococcus aureus from murine lungs. Infect Immun. 1982 Jun;36(3):1185–1191. doi: 10.1128/iai.36.3.1185-1191.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Yamamoto M., Yoshida T., Ide M., Matsumoto K. Opsonic activity of plasma fibronectin for Staphylococcus aureus by human alveolar macrophages: inefficacy of trypsin-sensitive staphylococcal fibronectin receptor. Tohoku J Exp Med. 1986 May;149(1):95–102. doi: 10.1620/tjem.149.95. [DOI] [PubMed] [Google Scholar]
- Onofrio J. M., Toews G. B., Lipscomb M. F., Pierce A. K. Granulocyte-alveolar-macrophage interaction in the pulmonary clearance of Staphylococcus aureus. Am Rev Respir Dis. 1983 Mar;127(3):335–341. doi: 10.1164/arrd.1983.127.3.335. [DOI] [PubMed] [Google Scholar]
- Pierce A. K., Reynolds R. C., Harris G. D. Leukocytic response to inhaled bacteria. Am Rev Respir Dis. 1977 Oct;116(4):679–684. doi: 10.1164/arrd.1977.116.4.679. [DOI] [PubMed] [Google Scholar]
- Pollock M. E., Bonner S. V. Comparison of undefined medium and its dialyzable fraction for growth of Mycoplasma. J Bacteriol. 1969 Feb;97(2):522–525. doi: 10.1128/jb.97.2.522-525.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G. B., Gross G. N., Pierce A. K. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am Rev Respir Dis. 1979 Sep;120(3):559–566. doi: 10.1164/arrd.1979.120.3.559. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Vial W. C., Hansen E. J. Role of C5 and recruited neutrophils in early clearance of nontypable Haemophilus influenzae from murine lungs. Infect Immun. 1985 Oct;50(1):207–212. doi: 10.1128/iai.50.1.207-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Grubbs B. Role of natural killer cells in infection with the mouse pneumonitis agent (murine Chlamydia trachomatis). Infect Immun. 1987 Jan;55(1):223–226. doi: 10.1128/iai.55.1.223-226.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



