Abstract
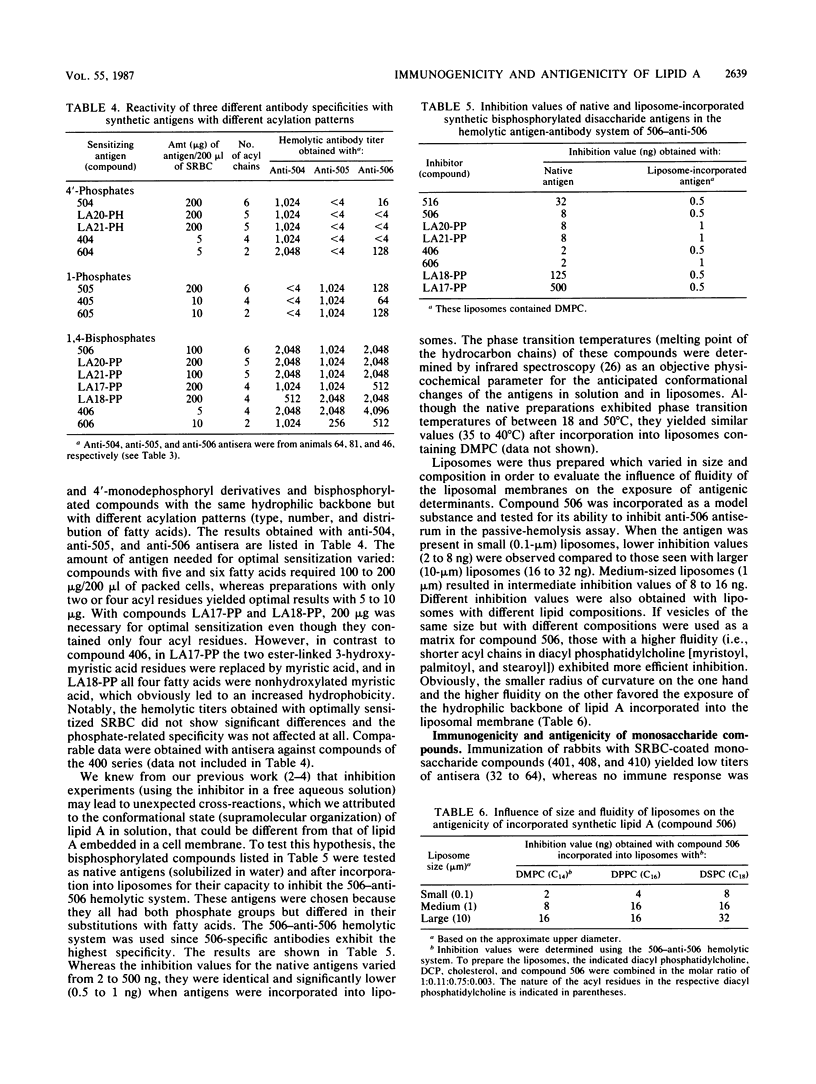
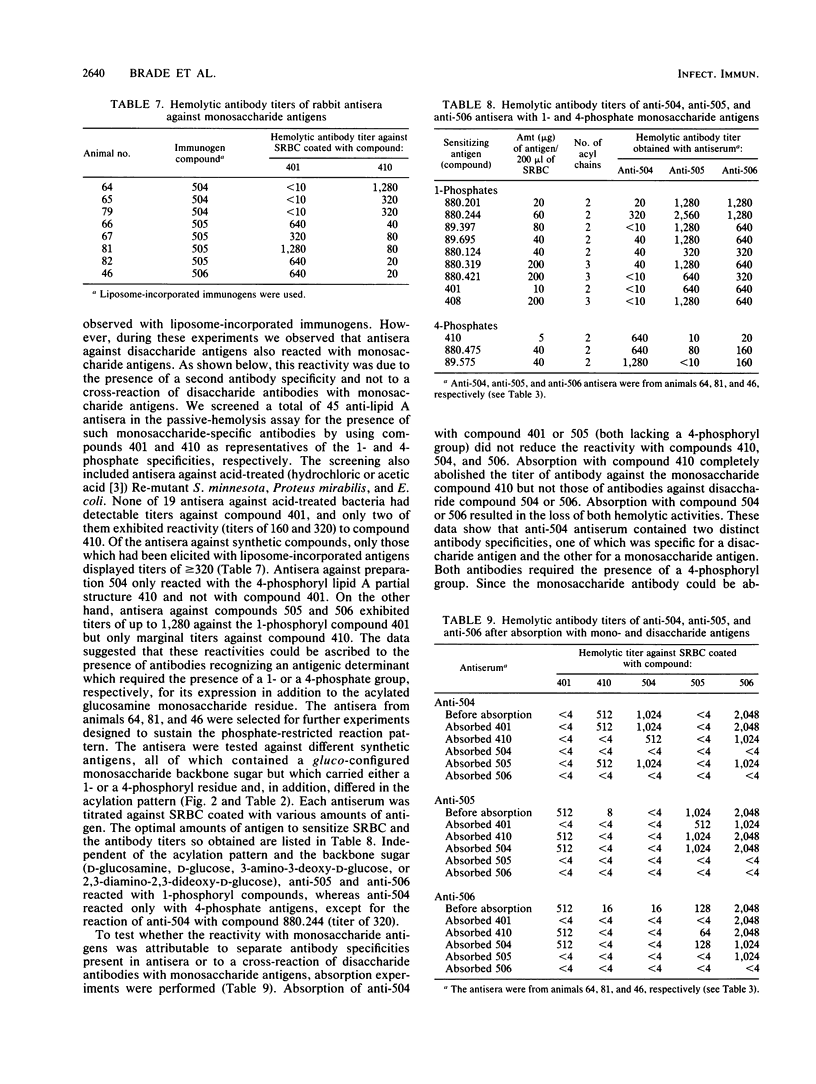
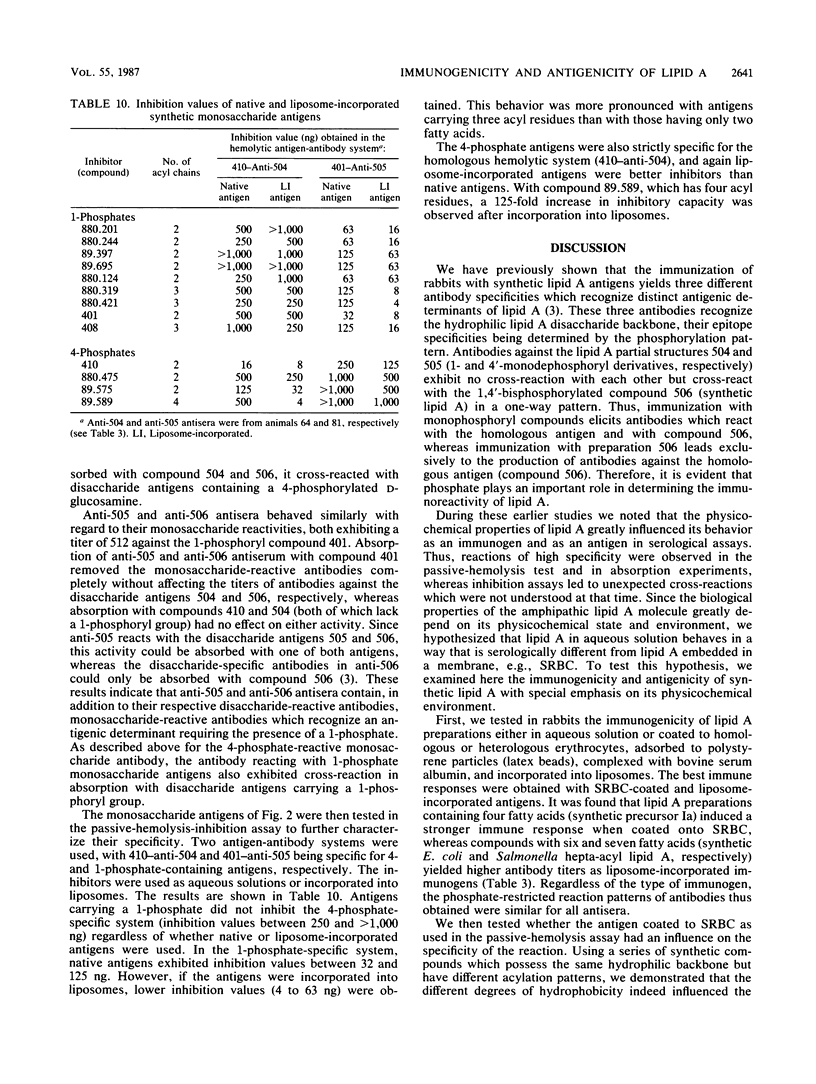
We investigated the immunogenicity and antigenicity of synthetic lipid A and partial structures thereof. Included in the study were compounds which varied in the position of phosphate (1-mono-, 4'-mono-, and 1,4'-bisphosphates) and in the acylation (type, number, and distribution of fatty acids) and, in the case of monosaccharide compounds, the nature of the backbone sugar (D-glucosamine, D-glucose, 3-amino-3-deoxy-D-glucose, and 2,3-diamino-2,3-dideoxy-D-glucose). With the aid of the passive-hemolysis and passive-hemolysis-inhibition assays and by absorption experiments, five distinct antibody specificities were detected in polyclonal rabbit antisera raised against sheep erythrocyte-coated lipid A and lipid A incorporated into the membrane of liposomes (liposome-incorporated immunogens). Three antibody specificities reacted with disaccharide antigens specific for a 1-mono-, 4'-mono-, and 1,4'-bisphosphorylated beta-1,6-linked D-glucosamine disaccharide. Two antibodies reacted with either 1- or 4-phosphates of acylated D-gluco-configured monosaccharides and exhibited no cross-reaction with each other. However, they cross-reacted with disaccharide antigens with phosphate groups in the appropriate positions. We found that the physicochemical state and the environment of lipid A modulated its immunoreactivity. The immunogenicity was best expressed by erythrocyte-coated and liposome-incorporated immunogens. The antigenicity of lipid A was also greatly influenced by its physical surroundings. The reaction pattern of the above antibodies was highly specific in the hemolysis assay and in absorption experiments (the antibody reacted with antigen embedded in a cell membrane), whereas some cross-reactivities were observed in inhibition studies (the antibody reacts with antigen in aqueous solution). By using liposome-incorporated antigens as inhibitors, nonspecific reactions were avoided and specific ones were enhanced. Thus the antibodies described above against lipid A recognize epitopes in the hydrophilic backbone, the exposure of which depends on the intrinsic physicochemical properties of lipid A on the one hand and the physical environment on the other.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji B., Alving C. R. Lipid A from endotoxin: antigenic activities of purified fractions in liposomes. J Immunol. 1979 Dec;123(6):2558–2562. [PubMed] [Google Scholar]
- Brade L., Brade H. Characterization of two different antibody specificities recognizing distinct antigenic determinants in free lipid A of Escherichia coli. Infect Immun. 1985 Jun;48(3):776–781. doi: 10.1128/iai.48.3.776-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Rietschel E. T., Kusumoto S., Shiba T., Brade H. Immunogenicity and antigenicity of natural and synthetic Escherichia coli lipid A. Prog Clin Biol Res. 1987;231:75–91. [PubMed] [Google Scholar]
- Brade L., Rietschel E. T., Kusumoto S., Shiba T., Brade H. Immunogenicity and antigenicity of synthetic Escherichia coli lipid A. Infect Immun. 1986 Jan;51(1):110–114. doi: 10.1128/iai.51.1.110-114.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Alterations in rats in vivo of the chemical structure of lipopolysaccharide from Salmonella abortus equi. Eur J Biochem. 1985 Oct 15;152(2):353–359. doi: 10.1111/j.1432-1033.1985.tb09205.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Jay F., Nerkar D., Veleva K., Brade H., Strittmatter W. Immunogenic properties of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):546–552. doi: 10.1093/clinids/6.4.546. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Freudenberg M., Brade L., Schade U., Rietschel E. T., Kusumoto S., Shiba T. Biological activity of synthetic heptaacyl lipid A representing a component of Salmonella minnesota R595 lipid A. Eur J Biochem. 1986 Oct 1;160(1):55–59. doi: 10.1111/j.1432-1033.1986.tb09939.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Hagge T., Lehmann V., Seydel U., Lindner B., Zähringer U. Isolation and structural analysis of two lipid A precursors from a KDO deficient mutant of Salmonella typhimurium differing in their hexadecanoic acid content. Arch Microbiol. 1985 May;141(4):353–358. doi: 10.1007/BF00428849. [DOI] [PubMed] [Google Scholar]
- Kasai N., Arata S., Mashimo J., Okuda K., Aihara Y., Kotani S., Takada H., Shiba T., Kusumoto S., Imoto M. Synthetic Salmonella-type lipid A antigen with high serological specificity. Infect Immun. 1986 Jan;51(1):43–48. doi: 10.1128/iai.51.1.43-48.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Mattsby-Baltzer I., Kaijser B. Lipid A and anti-lipid A. Infect Immun. 1979 Mar;23(3):758–763. doi: 10.1128/iai.23.3.758-763.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan N. A., Newsome P. M., Cunnington P. G., Palmer G. H., Wilson M. E. Protection against gram-negative infections with antiserum to lipid A from Salmonella minnesota R595. Infect Immun. 1974 Dec;10(6):1195–1201. doi: 10.1128/iai.10.6.1195-1201.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann D., Schultz C., Born J., Labischinski H., Brandenburg K., von Busse G., Brade H., Seydel U. Investigations into the polymorphism of lipid A from lipopolysaccharides of Escherichia coli and Salmonella minnesota by Fourier-transform infrared spectroscopy. Eur J Biochem. 1987 Apr 1;164(1):159–169. doi: 10.1111/j.1432-1033.1987.tb11007.x. [DOI] [PubMed] [Google Scholar]
- Ng A. K., Chen C. L., Chang C. M., Nowotny A. Relationship of structure to function in bacterial endotoxins: serologically cross-reactive components and their effect on protection of mice against some gram-negative infections. J Gen Microbiol. 1976 May;94(1):107–116. doi: 10.1099/00221287-94-1-107. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., Munford R. S. Dephosphorylation of the lipid A moiety of Escherichia coli lipopolysaccharide by mouse macrophages. Infect Immun. 1987 Apr;55(4):974–978. doi: 10.1128/iai.55.4.974-978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Brade H., Brade L., Brandenburg K., Schade U., Seydel U., Zähringer U., Galanos C., Lüderitz O., Westphal O. Lipid A, the endotoxic center of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Prog Clin Biol Res. 1987;231:25–53. [PubMed] [Google Scholar]
- Rietschel E. T., Galanos C. Lipid A antiserum-mediated protection against lipopolysaccharide- and lipid A-induced fever and skin necrosis. Infect Immun. 1977 Jan;15(1):34–49. doi: 10.1128/iai.15.1.34-49.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux-Darrieulat F., Parant M., Chedid L. Prevention of endotoxin-induced abortion by treatment of mice with antisera. J Infect Dis. 1978 Jan;137(1):7–13. doi: 10.1093/infdis/137.1.7. [DOI] [PubMed] [Google Scholar]


