Abstract
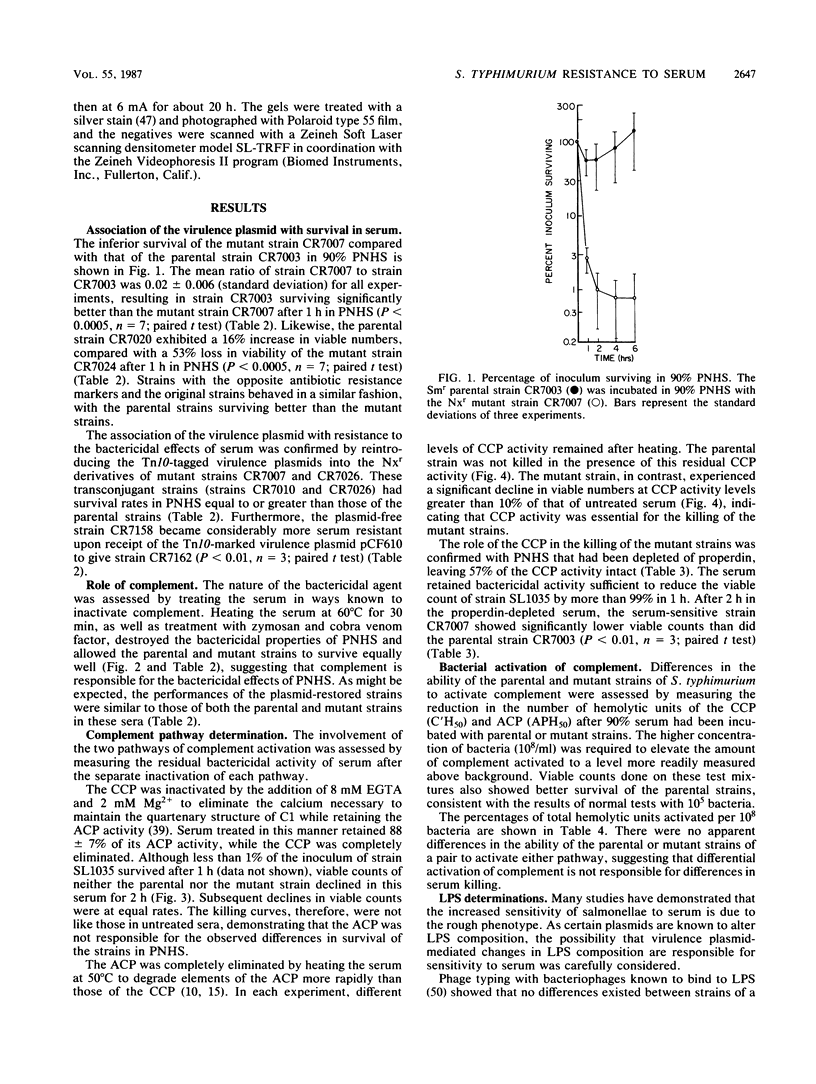
The association of the virulence plasmid of Salmonella typhimurium with resistance to the bactericidal activity of human serum was studied in chromosomally isogenic pairs of strains differing in their virulence plasmid status. The presence of the plasmid correlated in three pairs of strains with resistance to serum. The absence of the plasmid correlated with increased sensitivity to serum, whereas the reintroduction of the plasmid to the cell resulted in the restoration of resistance to serum. Complement was activated by the classical and alternative pathways equally well by both strains of a pair, but the differential bactericidal action of serum was apparent only after the activation of complement by the classical pathway. No differences in the chemical compositions or in the molecular weight ranges of the lipopolysaccharides were apparent between paired strains. This work confirms the presence of a virulence plasmid-associated mechanism of resistance to serum and distinguishes it from lipopolysaccharide-mediated resistance.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornson A. B., Bjornson H. S. Activation of complement by opportunist pathogens and chemotypes of Salmonella minnesota. Infect Immun. 1977 Jun;16(3):748–753. doi: 10.1128/iai.16.3.748-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Antibacterial peptide from normal rabbit serum. 2. Compositional microanalysis. Biochemistry. 1981 Oct 13;20(21):5981–5987. doi: 10.1021/bi00524a009. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Antibacterial peptide from normal rat serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry. 1981 Oct 13;20(21):5973–5981. doi: 10.1021/bi00524a008. [DOI] [PubMed] [Google Scholar]
- Clas F., Loos M. Antibody-independent binding of the first component of complement (C1) and its subcomponent C1q to the S and R forms of Salmonella minnesota. Infect Immun. 1981 Mar;31(3):1138–1144. doi: 10.1128/iai.31.3.1138-1144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clas F., Loos M. Killing of the S and Re forms of Salmonella minnesota via the classical pathway of complement activation in guinea-pig and human sera. Immunology. 1980 Aug;40(4):547–556. [PMC free article] [PubMed] [Google Scholar]
- Derylo M., Glowacka M., Lorkiewicz Z., Russa R. Plasmid-determined alterations of Salmonella typhimurium lipopolysaccharides. Mol Gen Genet. 1975 Sep 29;140(2):175–181. doi: 10.1007/BF00329785. [DOI] [PubMed] [Google Scholar]
- Dlabac V. The sensitivity of smooth and rough mutants of Salmonella typhimurium to bactericidal and bacteriolytic action of serum, lysozyme and to phagocytosis. Folia Microbiol (Praha) 1968;13(5):439–449. doi: 10.1007/BF02869196. [DOI] [PubMed] [Google Scholar]
- Donaldson D. M., Roberts R. R., Larsen H. S., Tew J. G. Interrelationship between serum beta-lysin, lysozyme, and the antibody-complement system in killing Escherichia coli. Infect Immun. 1974 Sep;10(3):657–666. doi: 10.1128/iai.10.3.657-666.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidinger D., Bello E., Mates A. The heterocytotoxicity of human serum. I. Activation of the alternative complement pathway by heterologous target cells. Cell Immunol. 1977 Mar 1;29(1):174–186. doi: 10.1016/0008-8749(77)90286-6. [DOI] [PubMed] [Google Scholar]
- Galdiero F., Tufano M. A., Sommese L., Folgore A., Tedesco F. Activation of complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984 Nov;46(2):559–563. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Stocker B. A. Transduction by bacteriophage P22 in nonsmooth mutants of Salmonella typhimurium. J Bacteriol. 1967 May;93(5):1588–1597. doi: 10.1128/jb.93.5.1588-1597.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Goodkofsky I., Lepow I. H. Functional relationship of factor B in the properdin system to C3 proactivator of human serum. J Immunol. 1971 Oct;107(4):1200–1204. [PubMed] [Google Scholar]
- Hackett J., Wyk P., Reeves P., Mathan V. Mediation of serum resistance in Salmonella typhimurium by an 11-kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis. 1987 Mar;155(3):540–549. doi: 10.1093/infdis/155.3.540. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bunge C., Hoog B., Steinbeck A., Bulling E. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infect Immun. 1985 Apr;48(1):175–182. doi: 10.1128/iai.48.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B., Kjelleberg S., Marshall K. C. Responses of marine bacteria under starvation conditions at a solid-water interface. Appl Environ Microbiol. 1983 Jan;45(1):43–47. doi: 10.1128/aem.45.1.43-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hawiger A., Gelfand J. A. A study of optimal reaction conditions for an assay of the human alternative complement pathway. Am J Clin Pathol. 1983 Jan;79(1):65–72. doi: 10.1093/ajcp/79.1.65. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai N., Nowotny A. Endotoxic glycolipid from a heptoseless mutant of Salmonella minnesota. J Bacteriol. 1967 Dec;94(6):1824–1836. doi: 10.1128/jb.94.6.1824-1836.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J Immunol. 1983 Apr;130(4):1867–1870. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Saxén H., Mäkelä P. H., Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983 Aug;41(2):563–569. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro M. A., Bitter-Suermann D., Timmis J. K., Agüero M. E., Cabello F. C., Sanyal S. C., Timmis K. N. traT gene sequences, serum resistance and pathogenicity-related factors in clinical isolates of Escherichia coli and other gram-negative bacteria. J Gen Microbiol. 1985 Jun;131(6):1511–1521. doi: 10.1099/00221287-131-6-1511. [DOI] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. E., Stocker B. A., Roantree R. J. Mapping of genes determining penicillin-resistance and serum-sensitivity in Salmonella enteritidis. J Gen Microbiol. 1980 Jun;118(2):367–376. doi: 10.1099/00221287-118-2-367. [DOI] [PubMed] [Google Scholar]
- Nelson B. W., Roantree R. J. Analyses of lipopolysaccharides extracted from penicillin-resistant, serum-sensitive salmonella mutants. J Gen Microbiol. 1967 Aug;48(2):179–188. doi: 10.1099/00221287-48-2-179. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Ross S. C., Densen P. Opsonophagocytosis of Neisseria gonorrhoeae: interaction of local and disseminated isolates with complement and neutrophils. J Infect Dis. 1985 Jan;151(1):33–41. doi: 10.1093/infdis/151.1.33. [DOI] [PubMed] [Google Scholar]
- Sansano M., Jr, Reynard A. M., Cunningham R. K. Inhibition of serum bactericidal reaction by lipopolysaccharide. Infect Immun. 1985 Jun;48(3):759–762. doi: 10.1128/iai.48.3.759-762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Casey S. G., Spitznagel J. K. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophil granulocytes. Infect Immun. 1984 Mar;43(3):834–838. doi: 10.1128/iai.43.3.834-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M. L., Specter S., Rodrick G. E., Friedman H. Differential complement activation and susceptibility to human serum bactericidal action by Vibrio species. Infect Immun. 1983 Dec;42(3):1187–1190. doi: 10.1128/iai.42.3.1187-1190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Parton R. A protein factor associated with serum resistance in Escherichia coli. J Med Microbiol. 1977 May;10(2):225–232. doi: 10.1099/00222615-10-2-225. [DOI] [PubMed] [Google Scholar]
- Tee G. L., Scott G. K. Analysis of outer membrane components of Escherichia coli ML308 225 and of a serum-resistant mutant. Infect Immun. 1980 May;28(2):387–392. doi: 10.1128/iai.28.2.387-392.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Sarvas M. Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):664–667. doi: 10.1128/jb.139.2.664-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Hutzler M., Kao L. Environmental modulation of lipopolysaccharide chain length alters the sensitivity of Escherichia coli to the neutrophil bactericidal/permeability-increasing protein. Infect Immun. 1986 Feb;51(2):594–599. doi: 10.1128/iai.51.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]




