Abstract
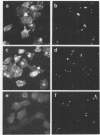
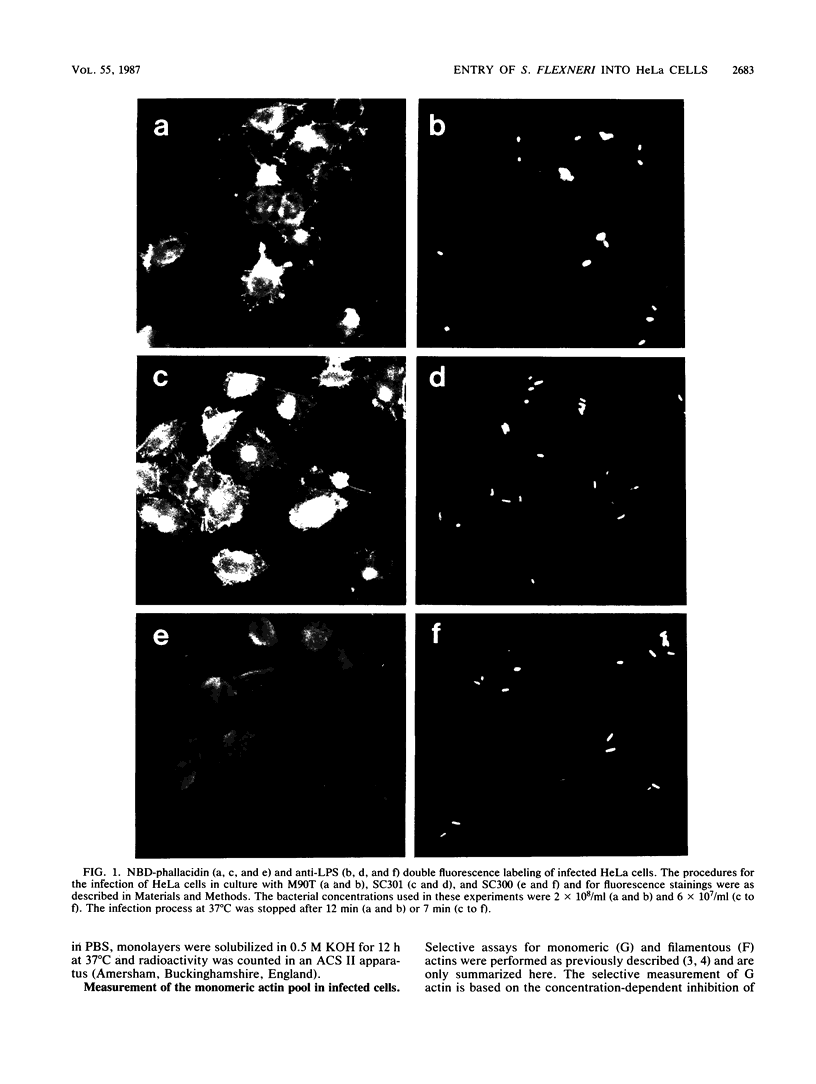
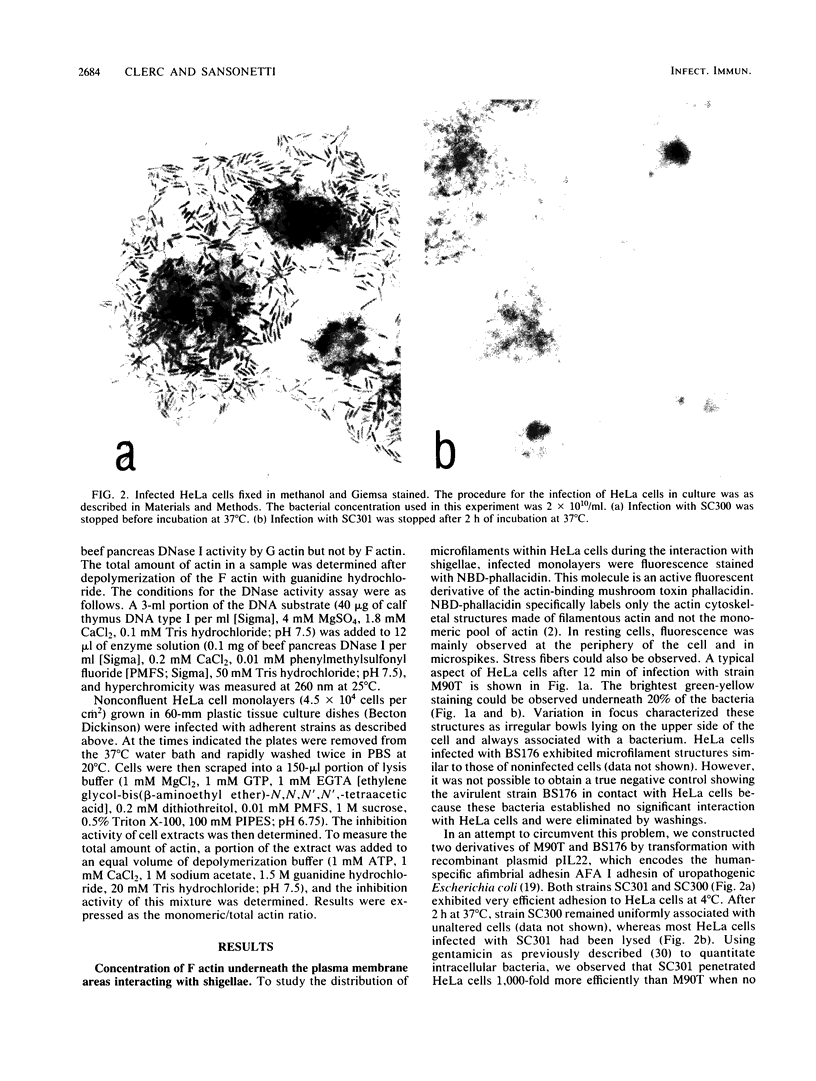
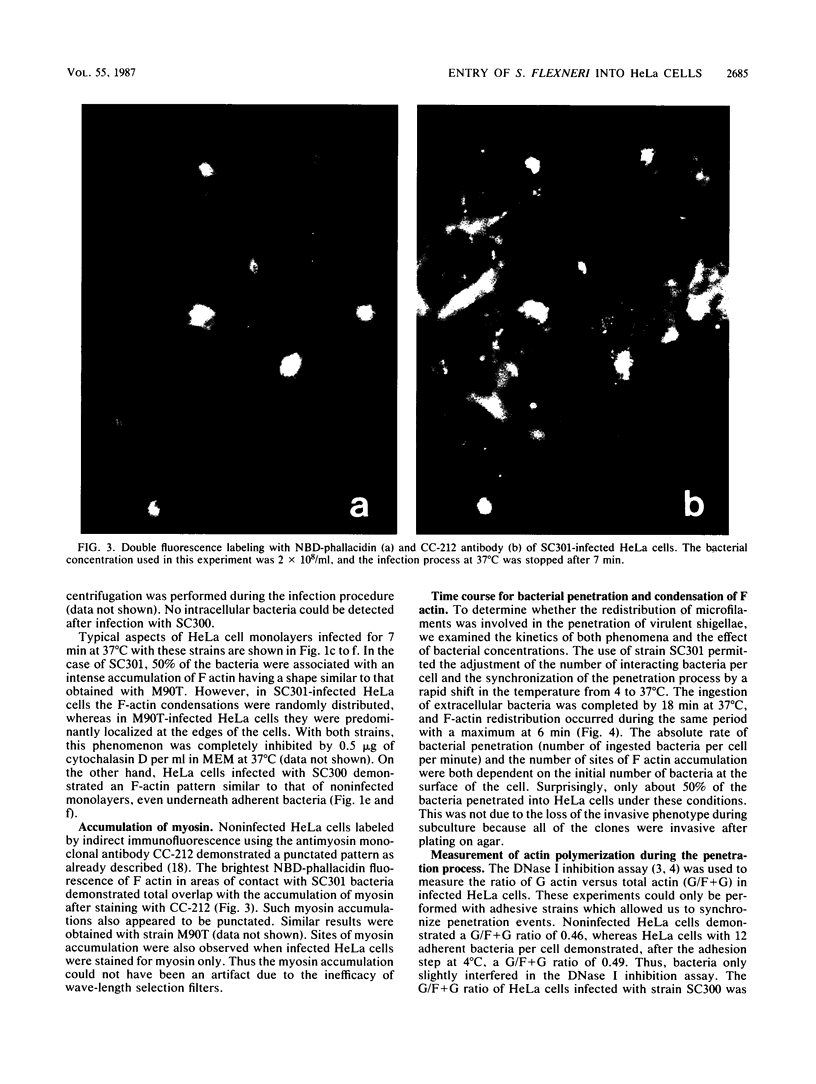
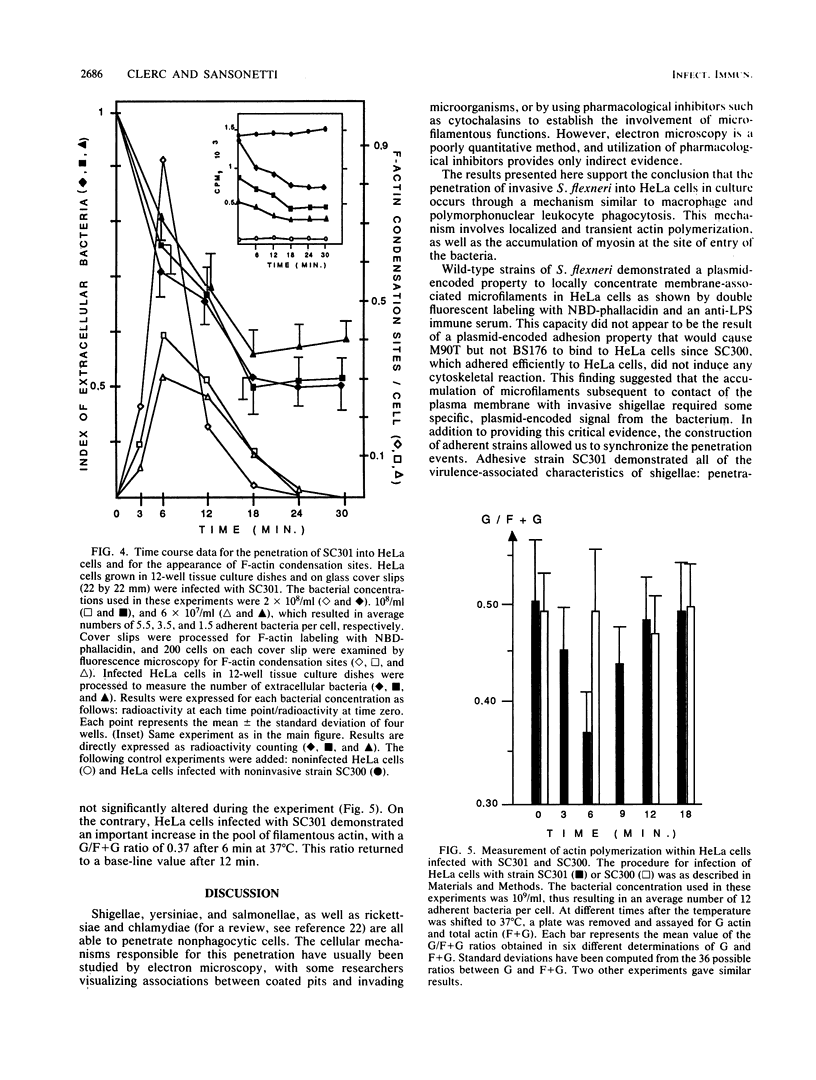
The enteroinvasive bacterium Shigella flexneri expresses a plasmid-mediated capacity to penetrate into nonphagocytic cells. By using 7-nitrobenz-2-oxa-1,3-diazole-phallacidin (NBD-phallacidin), a fluorescent dye which specifically stains microfilaments, we observed condensations of filamentous actin underneath the plasma membrane of HeLa cells which interacted with the invasive isolate M90T. As demonstrated by indirect immunofluorescence with the antimyosin monoclonal antibody CC-212, myosin accumulated at the same sites. The entry process could be synchronized by using strain SC301, a pIL22 transformant of M90T. pIL22, a recombinant plasmid encoding the Escherichia coli afimbrial adhesin AFA I, rendered shigellae highly adherent to HeLa cells. Using such a system, we demonstrated that the occurrence of bacterial penetration and the appearance of structures brightly stained by NBD-phallacidin were simultaneous events. Such microfilamentous structures resulted from de novo polymerization of the monomeric actin pool in a DNase I inhibition assay, as shown by measurement of the monomeric versus total actin content of infected HeLa cells. These data provide direct evidence that the penetration of S. flexneri into HeLa cells occurs through a mechanism similar to phagocytosis by professional phagocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J Cell Biol. 1982 Sep;94(3):613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Carlsson L. On the dynamics of the microfilament system in HeLa cells. J Cell Biol. 1982 Apr;93(1):122–128. doi: 10.1083/jcb.93.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Boyles J., Bainton D. F. Changes in plasma-membrane-associated filaments during endocytosis and exocytosis in polymorphonuclear leukocytes. Cell. 1981 Jun;24(3):905–914. doi: 10.1016/0092-8674(81)90116-1. [DOI] [PubMed] [Google Scholar]
- Clerc P. L., Ryter A., Mounier J., Sansonetti P. J. Plasmid-mediated early killing of eucaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect Immun. 1987 Mar;55(3):521–527. doi: 10.1128/iai.55.3.521-527.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. Lectin-mediated attachment and ingestion of yeast cells and erythrocytes by hamster fibroblasts. Exp Cell Res. 1977 Feb;104(2):325–334. doi: 10.1016/0014-4827(77)90098-2. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Silverstein S. C. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976 Sep 1;144(3):788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Cytotoxicity of Shigella dysenteriae 1 for cultured mammalian cells. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2485–2490. doi: 10.1093/ajcn/33.11.2485. [DOI] [PubMed] [Google Scholar]
- Hale T. L. Invasion of epithelial cells by shigellae. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):311–314. doi: 10.1016/s0769-2609(86)80040-0. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Wyrick P. B. Ultrastructural study of mode of entry of Chlamydia psittaci into L-929 cells. Infect Immun. 1986 Dec;54(3):855–863. doi: 10.1128/iai.54.3.855-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Inui K., Takano M., Hori R. Distinction of three types of D-glucose transport systems in animal cells. Biochem Biophys Res Commun. 1985 Oct 30;132(2):490–496. doi: 10.1016/0006-291x(85)91160-x. [DOI] [PubMed] [Google Scholar]
- Klotz C., Bordes N., Laine M. C., Sandoz D., Bornens M. Myosin at the apical pole of ciliated epithelial cells as revealed by a monoclonal antibody. J Cell Biol. 1986 Aug;103(2):613–619. doi: 10.1083/jcb.103.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Whisenand J., McIntosh A. T. Effects of cytochalasin B on actin and myosin association with particle binding sites in mouse macrophages: implications with regard to the mechanism of action of the cytochalasins. J Cell Biol. 1981 Nov;91(2 Pt 1):373–384. doi: 10.1083/jcb.91.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E. P., Axline S. G. Subplasmalemmal microfilaments and microtubules in resting and phagocytizing cultivated macrophages. J Cell Biol. 1973 Oct;59(1):12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Richards R. C. Fc receptor-directed phagocytic stimuli induce transient actin assembly at an early stage of phagocytosis in neutrophil leukocytes. Eur J Cell Biol. 1984 May;34(1):80–87. [PubMed] [Google Scholar]
- Stendahl O. I., Hartwig J. H., Brotschi E. A., Stossel T. P. Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol. 1980 Feb;84(2):215–224. doi: 10.1083/jcb.84.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D. I., Crawford N. Redistribution of membrane-bound and cytosolic action in rabbit polymorphonuclear leucocytes during phagocytosis. Biochem J. 1985 Feb 1;225(3):807–814. doi: 10.1042/bj2250807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]
- Wang K., Feramisco J. R., Ash J. F. Fluorescent localization of contractile proteins in tissue culture cells. Methods Enzymol. 1982;85(Pt B):514–562. doi: 10.1016/0076-6879(82)85050-7. [DOI] [PubMed] [Google Scholar]