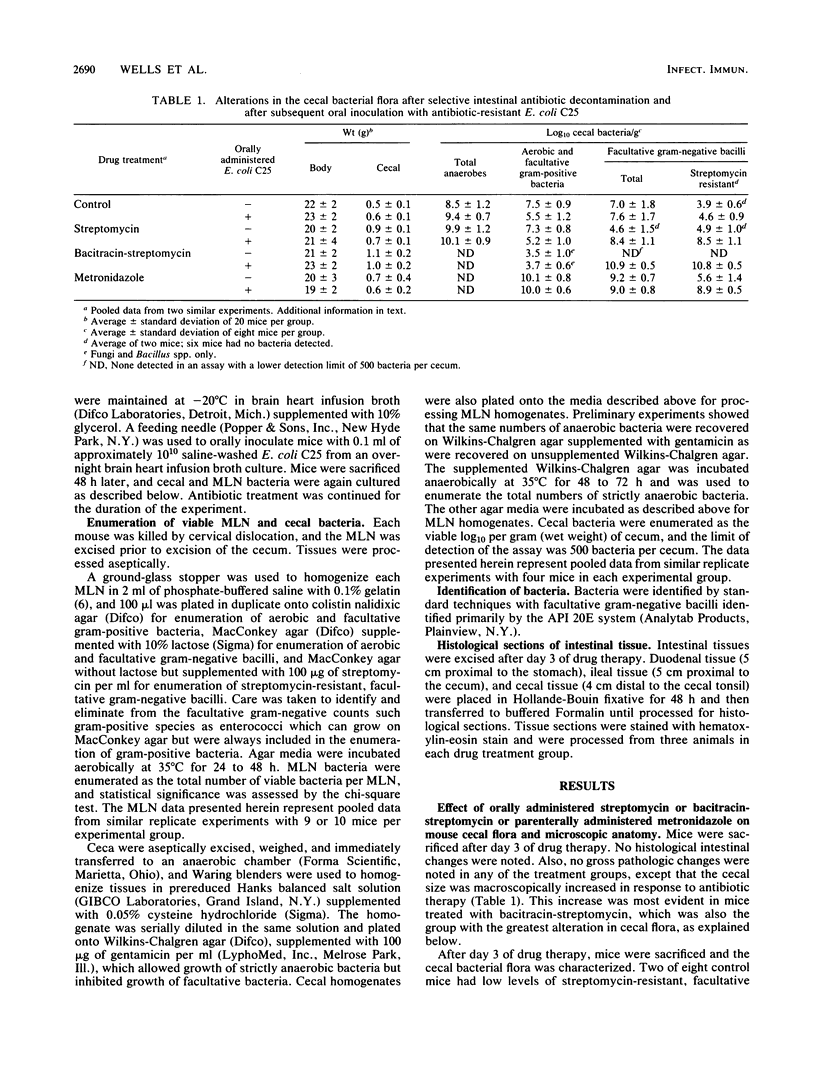
Abstract
It is thought that the normal enteric microflora acts not only to prevent intestinal colonization but also to prevent subsequent systemic dissemination of ingested, potentially pathogenic bacteria. To determine the relative roles of specific components of the intestinal bacterial flora in bacterial translocation out of the gut, mice were given various antimicrobial agents to selectively eliminate specific groups of intestinal bacteria. The cecal flora and the translocating bacteria in mesenteric lymph nodes were monitored both before and after oral inoculation with antibiotic-resistant Escherichia coli C25. Orally administered streptomycin selectively eliminated cecal facultative gram-negative bacilli, orally administered bacitracin-streptomycin eliminated all cecal bacterial species except low numbers of aerobic sporeformers, and parenterally administered metronidazole selectively eliminated cecal anaerobic bacteria. Compared with control mice, only metronidazole-treated mice had significantly increased rates of dissemination of intestinal bacteria into mesenteric lymph nodes, indicating that the exclusive absence of anaerobic bacteria facilitated the translocation of the intestinal facultative bacteria. In a parallel experiment with streptomycin-resistant E. coli C25 as a marker, parallel results were obtained. Metronidazole increased the translocation of the marker strain and the indigenous strains of intestinal bacteria. Thus, anaerobes appeared to play a key role in confining indigenous bacteria to the gut. However, intestinal colonization and translocation of E. coli C25 occurred most readily after bacitracin-streptomycin treatment, suggesting that in addition to anaerobic bacteria, other bacterial groups may play a role in limiting the intestinal colonization and extraintestinal dissemination of E. coli C25.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect Immun. 1981 Sep;33(3):854–861. doi: 10.1128/iai.33.3.854-861.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker A. W., Rozenberg-Arska M., Sixma J. J., Verhoef J. Prevention of infection by trimethoprim-sulfamethoxazole plus amphotericin B in patients with acute nonlymphocytic leukaemia. Ann Intern Med. 1981 Nov;95(5):555–559. doi: 10.7326/0003-4819-95-5-555. [DOI] [PubMed] [Google Scholar]
- Ducluzeau R., Ladire M., Callut C., Raibaud P., Abrams G. D. Antagonistic effect of extremely oxygen-sensitive clostridia from the microflora of conventional mice and of Escherichia coli against Shigella flexneri in the digestive tract of gnotobiotic mice. Infect Immun. 1977 Aug;17(2):415–424. doi: 10.1128/iai.17.2.415-424.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiot H. F., van der Meer J. W., van Furth R. Selective antimicrobial modulation of human microbial flora: infection prevention in patients with decreased host defense mechanisms by selective elimination of potentially pathogenic bacteria. J Infect Dis. 1981 May;143(5):644–654. doi: 10.1093/infdis/143.5.644. [DOI] [PubMed] [Google Scholar]
- Gurwith M., Truog K., Hinthorn D., Liu C. Trimethoprim-sulfamethoxazole and trimethoprim alone for prophylaxis of infection in granulocytopenic patients. Rev Infect Dis. 1982 Mar-Apr;4(2):593–601. doi: 10.1093/clinids/4.2.593. [DOI] [PubMed] [Google Scholar]
- Hargadon M. T., Young V. M., Schimpff S. C., Wade J. C., Minah G. E. Selective suppression of alimentary tract microbial flora as prophylaxis during granulocytopenia. Antimicrob Agents Chemother. 1981 Nov;20(5):620–624. doi: 10.1128/aac.20.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. J., Stein A. J., Casey S. W., Que J. U. Protective role of intestinal flora against infection with Pseudomonas aeruginosa in mice: influence of antibiotics on colonization resistance. Infect Immun. 1985 Jan;47(1):118–122. doi: 10.1128/iai.47.1.118-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985 Sep;49(3):654–663. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima K., Deitch E., Berg R. Promotion by burn stress of the translocation of bacteria from the gastrointestinal tracts of mice. Arch Surg. 1984 Feb;119(2):166–172. doi: 10.1001/archsurg.1984.01390140032006. [DOI] [PubMed] [Google Scholar]
- Maier B. R., Hentges D. J. Experimental Shigella infections in laboratory animals. I. Antagonism by human normal flora components in gnotobiotic mice. Infect Immun. 1972 Aug;6(2):168–173. doi: 10.1128/iai.6.2.168-173.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Louie T. J., Tally F. P., Bartlett J. G. Activity of metronidazole against Escherichia coli in experimental intra-abdominal sepsis. J Antimicrob Chemother. 1979 Mar;5(2):201–210. doi: 10.1093/jac/5.2.201. [DOI] [PubMed] [Google Scholar]
- Owens W. E., Berg R. D. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980 Feb;27(2):461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J. U., Hentges D. J. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infect Immun. 1985 Apr;48(1):169–174. doi: 10.1128/iai.48.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Intestinal beta-lactamase activity in ampicillin-induced, Clostridium difficile-associated ileocecitis. J Infect Dis. 1983 Feb;147(2):227–235. doi: 10.1093/infdis/147.2.227. [DOI] [PubMed] [Google Scholar]
- Steffen E. K., Berg R. D. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983 Mar;39(3):1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. C., de Jongh C. A., Newman K. A., Crowley J., Wiernik P. H., Schimpff S. C. Selective antimicrobial modulation as prophylaxis against infection during granulocytopenia: trimethoprim-sulfamethoxazole vs. nalidixic acid. J Infect Dis. 1983 Apr;147(4):624–634. doi: 10.1093/infdis/147.4.624. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Maddaus M. A., Simmons R. L. Role of the macrophage in the translocation of intestinal bacteria. Arch Surg. 1987 Jan;122(1):48–53. doi: 10.1001/archsurg.1987.01400130054008. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971 Sep;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk-van der Wees Colonization resistance of the digestive tract and the spread of bacteria to the lymphatic organs in mice. J Hyg (Lond) 1972 Jun;70(2):335–342. doi: 10.1017/s0022172400022385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis J. M., Lekkerkerk J. E. Colonization resistance of the digestive tract of mice during systemic antibiotic treatment. J Hyg (Lond) 1972 Dec;70(4):605–610. doi: 10.1017/s0022172400022464. [DOI] [PMC free article] [PubMed] [Google Scholar]


