Abstract
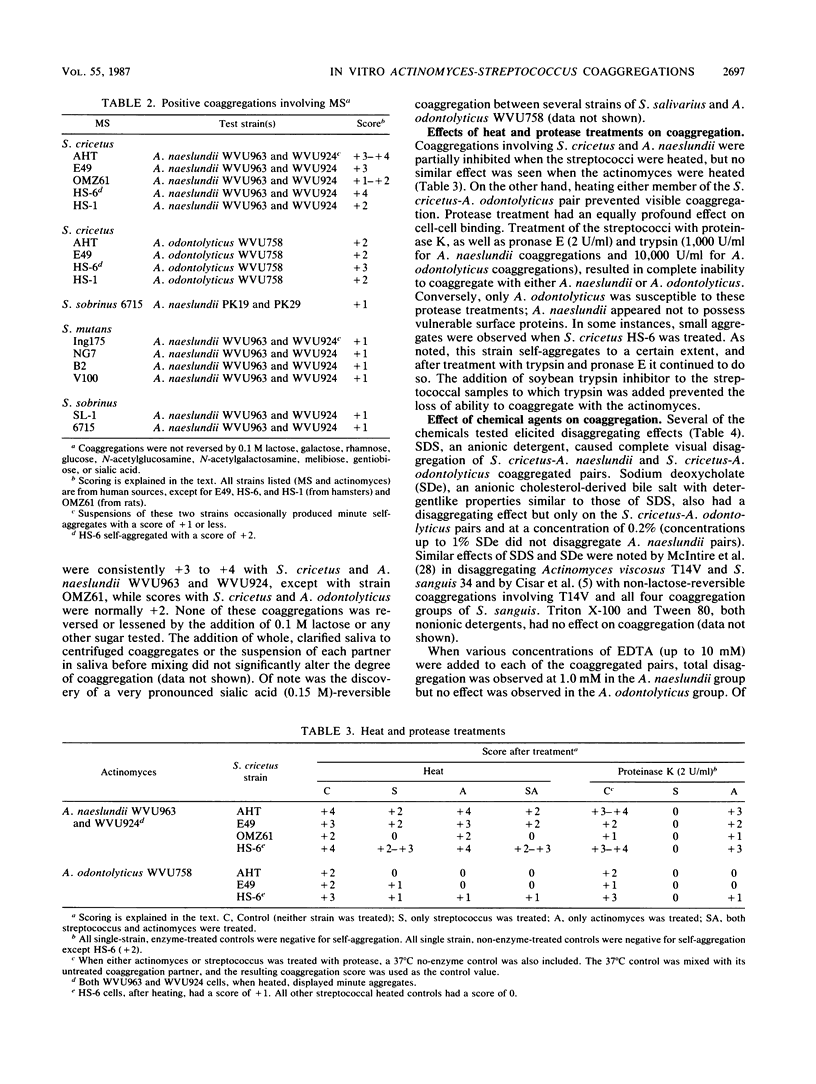
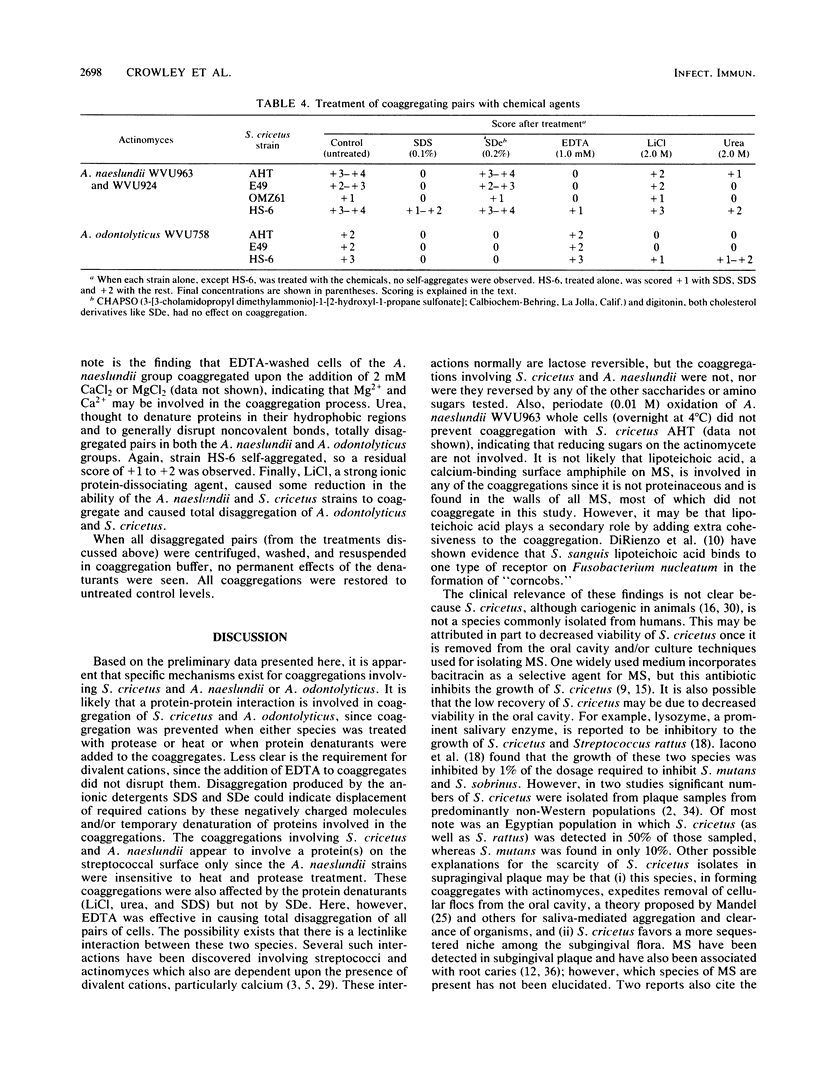
Mutans streptococci (MS) representing eight different serotypes were tested for their ability to coaggregate in vitro with oral actinomyces and other streptococcal species. Of the mutans streptococci tested, only strains of S. cricetus (formerly S. mutans serotype a) displayed pronounced coaggregations and only with certain strains of actinomyces. S. cricetus coaggregated, by lactose nonreversible mechanisms, with serotype 4 Actinomyces naeslundii WVU963 and WVU924 and with serotype 2 Actinomyces odontolyticus WVU758. The first pair was disaggregated by protein denaturants (e.g., sodium dodecyl sulfate and urea) and EDTA. This coaggregation was inhibited when the streptococcal, but not the actinomyces, partner was pretreated with either heat or protease, suggesting the presence of a protein mediator on only the streptococcal cell surface. The S. cricetus-A. odontolyticus coaggregation appeared to involve protein components on each cell, as shown by the lack of coaggregation after pretreatment of either cell type with heat or proteases. This coaggregation was also reversed by sodium dodecyl sulfate and urea, as well as by sodium deoxycholate, but not by EDTA. The data indicate that different mechanisms may be involved in each of these coaggregations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyar R. M., Bowden G. H. The microflora associated with the progression of incipient carious lesions of children living in a water-fluoridated area. Caries Res. 1985;19(4):298–306. doi: 10.1159/000260859. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of Streptococcus mutans strains in some selected areas of the world. Odontol Revy. 1972;23(4):401–410. [PubMed] [Google Scholar]
- Cisar J. O., Curl S. H., Kolenbrander P. E., Vatter A. E. Specific absence of type 2 fimbriae on a coaggregation-defective mutant of Actinomyces viscosus T14V. Infect Immun. 1983 May;40(2):759–765. doi: 10.1128/iai.40.2.759-765.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Sandberg A. L., Mergenhagen S. E. The function and distribution of different fimbriae on strains of Actinomyces viscosus and Actinomyces naeslundii. J Dent Res. 1984 Mar;63(3):393–396. doi: 10.1177/00220345840630030701. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Banting D. W., Fillery E. D. Streptococcus mutans and Lactobacillus detection in the assessment of dental root surface caries risk. J Dent Res. 1985 Oct;64(10):1245–1249. doi: 10.1177/00220345850640101301. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Ooshima T., Torii M., Imanishi H., Masuda N., Sobue S., Kotani S. Dental caries induction in experimental animals by clinical strains of Streptococcus mutans isolated from Japanese children. Microbiol Immunol. 1978;22(6):301–314. doi: 10.1111/j.1348-0421.1978.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Hill P. E., Knox K. W., Schamschula R. G., Tabua J. The identification and enumeration of actinomyces from plaque of New Guinea indigenes. Caries Res. 1977;11(6):327–335. doi: 10.1159/000260287. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Celesk R. A. Coaggregation of human oral Cytophaga species and Actinomyces israelii. Infect Immun. 1983 Jun;40(3):1178–1185. doi: 10.1128/iai.40.3.1178-1185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Inouye Y., Holdeman L. V. New Actinomyces and Streptococcus coaggregation groups among human oral isolates from the same site. Infect Immun. 1983 Aug;41(2):501–506. doi: 10.1128/iai.41.2.501-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Prevalence of viridans streptococci exhibiting lactose-inhibitable coaggregation with oral actinomycetes. Infect Immun. 1983 Aug;41(2):449–452. doi: 10.1128/iai.41.2.449-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Van der Hoeven J. S. Role of interbacterial adherence in colonization of the oral cavities of gnotobiotic rats infected with Streptococcus mutans and Veillonella alcalescens. Infect Immun. 1981 Aug;33(2):467–472. doi: 10.1128/iai.33.2.467-472.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E. Inhibitors of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34: beta-galactosides, related sugars, and anionic amphipathic compounds. Infect Immun. 1982 Apr;36(1):371–378. doi: 10.1128/iai.36.1.371-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Navia J. M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975 Jul;12(1):69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. H., Kleinman J. L. Effect of microbial interactions on in vitro plaque formation by Streptococcus mutans. J Dent Res. 1974 Mar-Apr;53(2):427–434. doi: 10.1177/00220345740530024201. [DOI] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J. Sucrose metabolism in resting-cell suspensions of caries associated and non-caries-associated dental plaque. Infect Immun. 1977 Jul;17(1):43–54. doi: 10.1128/iai.17.1.43-54.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J., Pape H. L., Jr, grenier E. Predominant cultivable flora isolated from human root surface caries plaque. Infect Immun. 1975 Apr;11(4):727–731. doi: 10.1128/iai.11.4.727-731.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., Jacobs T. Cell wall-associated protein antigens of Streptococcus salivarius: purification, properties, and function in adherence. Infect Immun. 1982 Oct;38(1):233–242. doi: 10.1128/iai.38.1.233-242.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Identification of a Streptococcus salivarius cell wall component mediating coaggregation with Veillonella alcalescens V1. Infect Immun. 1981 May;32(2):723–730. doi: 10.1128/iai.32.2.723-730.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson S. H., Svare C. W., Weber D. Load-bearing capacity of functioning alumina dental endosseous implants. J Dent Res. 1976 Jan-Feb;55(1):22–29. doi: 10.1177/00220345760550011801. [DOI] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]
- van Houte J., Duchin S. Streptococcus mutans in the mouths of children with congenital sucrase deficiency. Arch Oral Biol. 1975 Nov;20(11):771–773. doi: 10.1016/0003-9969(75)90050-3. [DOI] [PubMed] [Google Scholar]




