Abstract
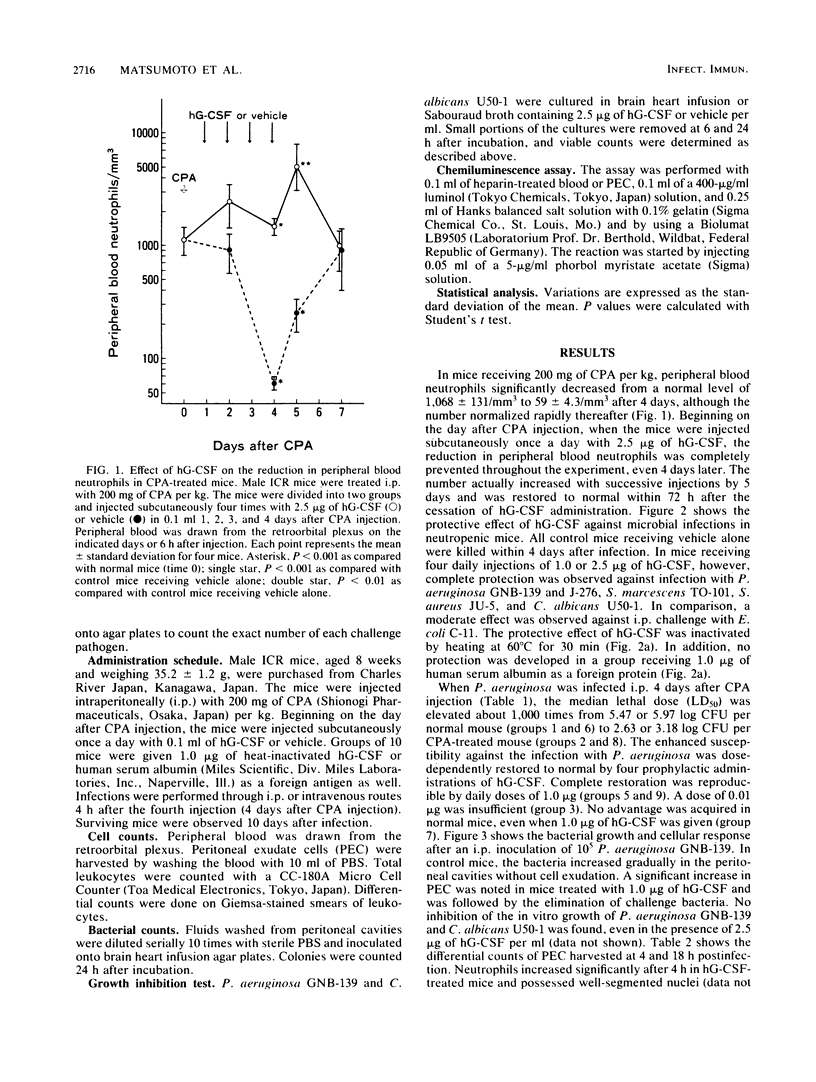
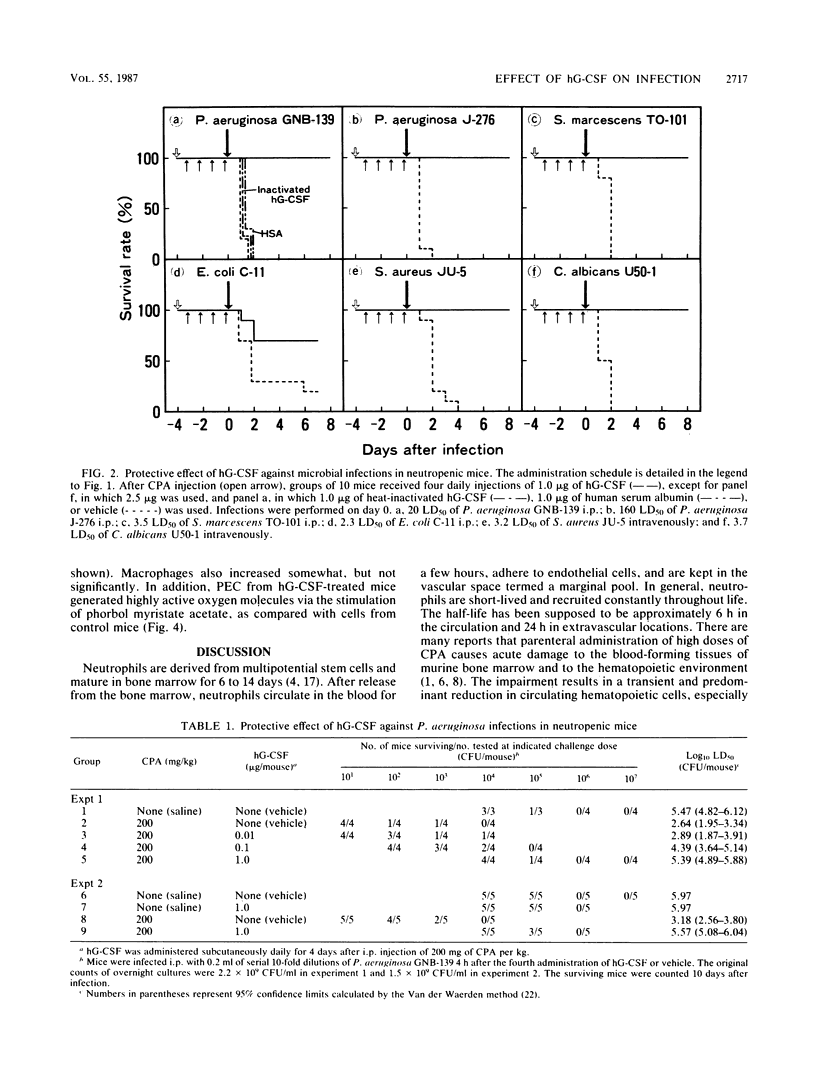
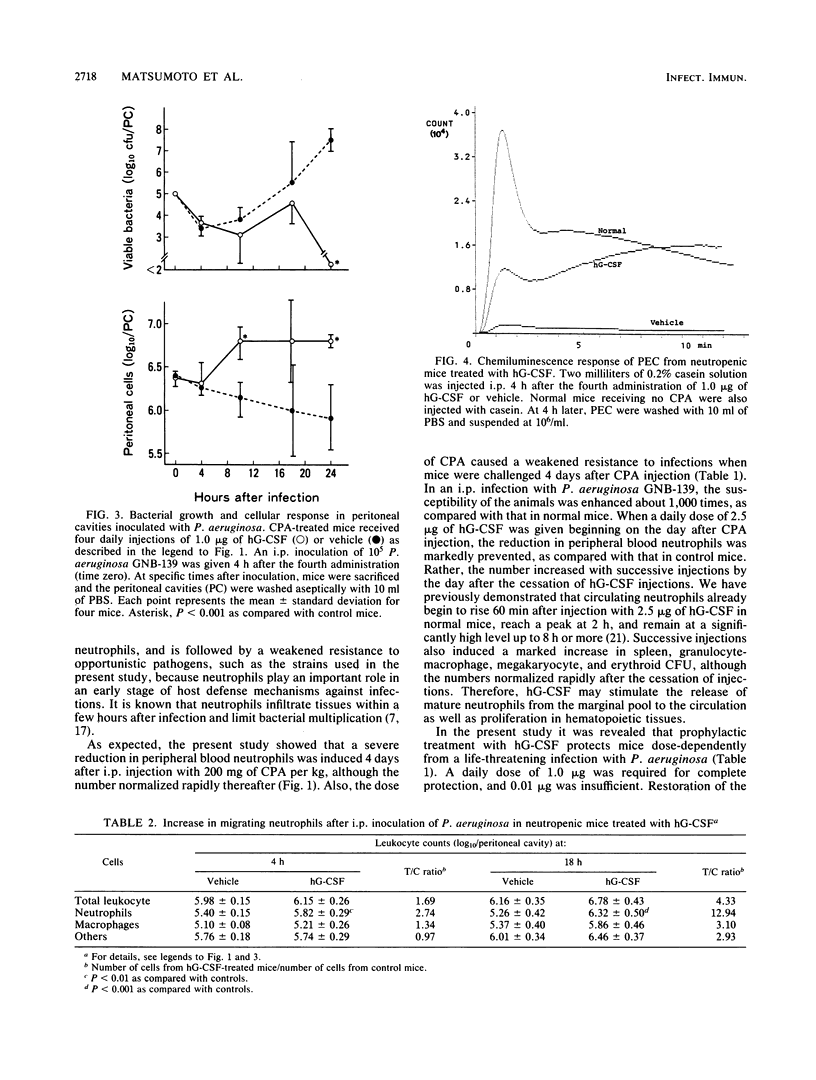
A purified human granulocyte colony-stimulating factor (hG-CSF) was studied for its protective effect on the induction of neutropenia and enhanced susceptibility to microbial infections in mice receiving cyclophosphamide (CPA). A severe reduction in peripheral blood neutrophils was induced 4 days after injection with 200 mg of CPA per kg although the level normalized rapidly thereafter. When mice were injected subcutaneously once a day with 2.5 micrograms of hG-CSF beginning on the day after CPA injection, the reduction was prevented markedly, even 4 days later. On the other hand, in mice receiving CPA 4 days prior to infection, a weakened resistance to intraperitoneal challenge with a strain of Pseudomonas aeruginosa was induced. This weakened resistance was dose-dependently restored to normal by four daily injections with hG-CSF. A daily dose of 1.0 microgram was required for complete restoration, although hG-CSF did not directly inhibit bacterial growth in vitro. In hG-CSF-treated mice, morphologically mature neutrophils migrated rapidly into the peritoneal cavities where bacteria were inoculated, followed by a rapid elimination of bacteria from the locality as compared with controls. In addition, the same treatment with hG-CSF was able to protect significantly against systemic infections caused by Serratia marcescens, Escherichia coli, Staphylococcus aureus, and Candida albicans. These data show the possibility that prophylactic therapy with hG-CSF may augment the resistance of immunocompromised patients to infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. S., Kuznetsky R. D. Effects of chemotherapeutic agents on hemopoietic stromal tissue: I. Effects of cyclophosphamide and bleomycin on hemopoiesis in subcutaneous femur implants. Exp Hematol. 1984 Mar;12(3):153–161. [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Rodriguez V., Chang H. Y., Narboni Fever and infection in leukemic patients: a study of 494 consecutive patients. Cancer. 1978 Apr;41(4):1610–1622. doi: 10.1002/1097-0142(197804)41:4<1610::aid-cncr2820410452>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Vigneulle R., Hellman S. Differential effects of cytotoxic agents on hematopoietic progenitors. Cancer Res. 1981 Jun;41(6):2338–2342. [PubMed] [Google Scholar]
- DeWys W. D., Goldin A., Man, El N. Hematopoietic recovery after large doses of cyclophosphamide: correlation of proliferative state with sensitivity. Cancer Res. 1970 Jun;30(6):1692–1697. [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- Furusawa S. Autoimmune neutropenia. Nihon Ketsueki Gakkai Zasshi. 1985 Dec;48(8):1753–1763. [PubMed] [Google Scholar]
- Maehara N., Ho M. Cellular origin of interferon induced by bacterial lipopolysaccharide. Infect Immun. 1977 Jan;15(1):78–83. doi: 10.1128/iai.15.1.78-83.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Nomura H., Imazeki I., Oheda M., Kubota N., Tamura M., Ono M., Ueyama Y., Asano S. Purification and characterization of human granulocyte colony-stimulating factor (G-CSF). EMBO J. 1986 May;5(5):871–876. doi: 10.1002/j.1460-2075.1986.tb04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G. Phagocytic cell dysfunction. J Allergy Clin Immunol. 1986 Mar;77(3):387–398. doi: 10.1016/0091-6749(86)90169-7. [DOI] [PubMed] [Google Scholar]
- Rubinstein S., Familletti P. C., Pestka S. Convenient assay for interferons. J Virol. 1981 Feb;37(2):755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Ryan J. L., McAdam K. P., Rosenstreich D. L. Detection of a mediator derived from endotoxin-stimulated macrohpages that induces the acute phase serum amyloid A response in mice. J Exp Med. 1979 Sep 19;150(3):597–606. doi: 10.1084/jem.150.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R., Gans P. J., McCarroll L. A. The synthesis and secretion of granulocyte-monocyte colony-stimulating activity (CSA) by isolated human monocytes: kinetics of the response to bacterial endotoxin. J Immunol. 1983 Feb;130(2):800–807. [PubMed] [Google Scholar]
- Xu C. X., Hendry J. H., Testa N. G. Residual deficiencies in hemopoietic precursor cell populations after repeated irradiation of mice with x-rays or neutrons: dose-response relationships. Exp Hematol. 1986 Mar;14(3):230–233. [PubMed] [Google Scholar]


