Abstract
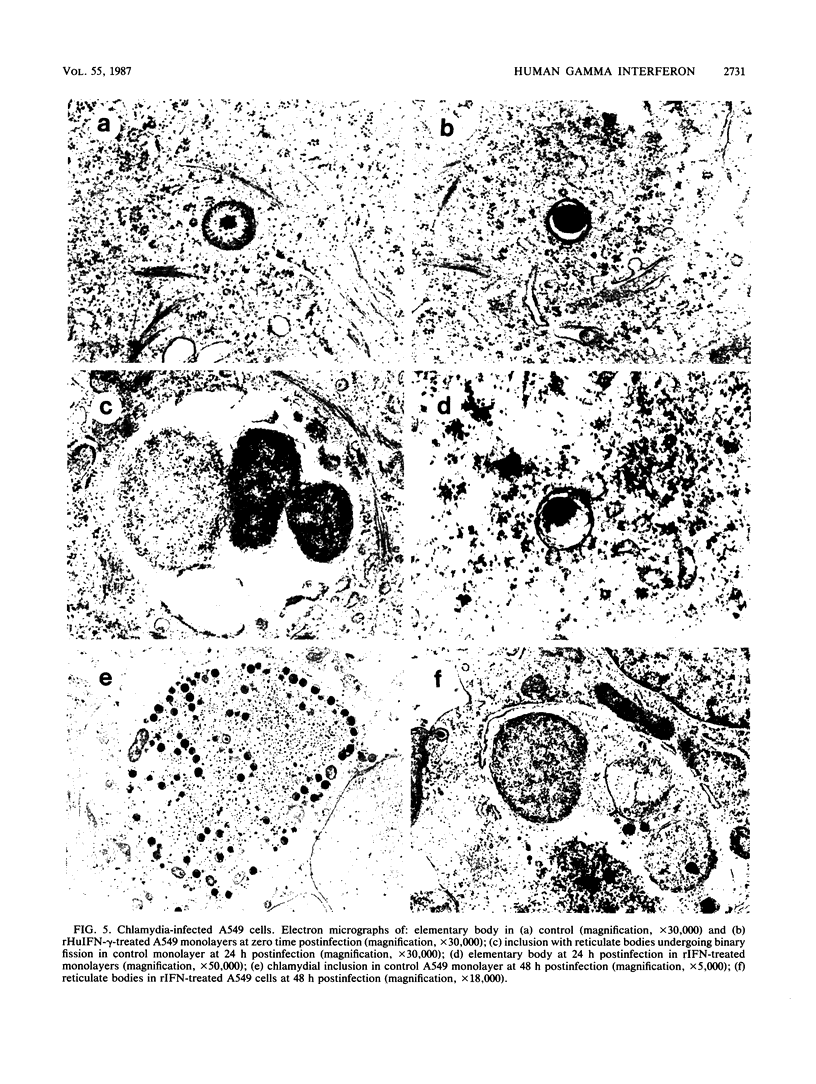
The effects of recombinant human gamma interferon (rHuIFN-gamma; two identical monomers of 140 residues in length) and of two re-engineered C-terminal variants, rHuIFN-gamma Tetra-Ser (residues 129 to 132 replaced by serine) and rHuIFN-gamma 125 (two identical monomers of 125 residues each with the last 14 residues plus an additional alanine from the C terminus deleted), were compared in terms of several in vitro biological activities. By using three different human cell lines (HeLa 229, HEp-2, and A549), the interferons were tested for their ability to inhibit: (i) growth of Chlamydia trachomatis; (ii) replication of encephalomyocarditis virus; and (iii) cell growth. rHuIFN-gamma restricted the growth of chlamydiae to 50% of the non-IFN-treated control at concentrations ranging from 0.01 to 0.05 ng/ml, depending on the cell type assayed. One of the modified proteins, rHuIFN-gamma Tetra-Ser, also decreased the growth of chlamydiae, but it required a concentration of approximately 0.5 ng/ml to produce 50% inhibition. rHuIFN-gamma 125 had the lowest antichlamydial activity of the three IFN-gamma variants tested; concentrations of 1 to 20 ng/ml were needed to reduce the growth of C. trachomatis to 50% of that of the control. The relative antiviral and antiproliferative activities of the three IFN-gamma preparations paralleled their antichlamydial activities in these three cell lines. The antiencephalomyocarditis virus activities of rHuIFN-gamma Tetra-Ser and rHuIFN-gamma 125 were reduced by approximately 10-fold and 10(2)- to 10(3)-fold, respectively, compared with the antiviral activity of rHuIFN-gamma. Proliferation of the three cell lines was restricted to approximately 50% of the control with 0.5 to 10 ng of rHuIFN-gamma per ml. Inhibition of cell growth by rHuIFN-gamma Tetra-Ser was significant only at concentrations equal to or greater than 30 ng/ml, and the rHuIFN-gamma 125 variant did not significantly decrease the growth of any of the three cell lines at the concentrations tested. These results suggest that the C-terminal portion of rHuIFN-gamma is critical for maintaining the conformation necessary for inducing the antichlamydial, antiviral, and antiproliferative activities of the molecule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Hsu Y. R., Parker C. G., Lai P. H. Role of polycationic C-terminal portion in the structure and activity of recombinant human interferon-gamma. J Biol Chem. 1986 Jun 25;261(18):8534–8539. [PubMed] [Google Scholar]
- Baron S., Weigent D., Stanton G. J., Peterson J. The protective role of endogenous interferon in viral, bacterial, and protozoal infections. Antiviral Res. 1985;Suppl 1:173–183. doi: 10.1016/s0166-3542(85)80026-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983 Dec;42(3):1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Kazar J., Gillmore J. D., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971 Jun;3(6):825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin S., Hahn T. Evaluation of the human interferon system in viral disease. Clin Exp Immunol. 1981 Dec;46(3):475–483. [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Characterization of Chlamydia DNA by restriction endonuclease cleavage. Infect Immun. 1983 Aug;41(2):604–608. doi: 10.1128/iai.41.2.604-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Sueltenfuss E. A., Pollard M. Cytochemical Assay of Interferon Produced by Duck Hepatitis Virus. Science. 1963 Feb 15;139(3555):595–596. doi: 10.1126/science.139.3555.595. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-Like Virus-Inhibitor Induced in Human Leukocytes by Phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. doi: 10.1126/science.149.3681.310. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Goebel J. M., Czarniecki C. W., Peterson E. M. Ultrastructural analysis of the growth cycle of Chlamydia trachomatis in mouse cells treated with recombinant human alpha-interferons. Exp Mol Pathol. 1984 Oct;41(2):227–235. doi: 10.1016/0014-4800(84)90039-x. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Fennie C. W., Czarniecki C. W. The anti-chlamydial and anti-proliferative activities of recombinant murine interferon-gamma are not dependent on tryptophan concentrations. J Immunol. 1985 Dec;135(6):4198–4200. [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M. Genital infections. Med Clin North Am. 1983 Sep;67(5):1059–1073. doi: 10.1016/s0025-7125(16)31166-x. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Goebel J. M., Fennie C. W., Czarniecki C. W. Interferon-induced inhibition of Chlamydia trachomatis: dissociation from antiviral and antiproliferative effects. Infect Immun. 1985 Mar;47(3):719–722. doi: 10.1128/iai.47.3.719-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M. Scanning electron microscopy of McCoy cells infected with Chlamydia trachomatis. Exp Mol Pathol. 1982 Apr;36(2):217–226. doi: 10.1016/0014-4800(82)90095-8. [DOI] [PubMed] [Google Scholar]