Abstract
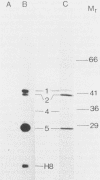
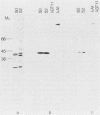
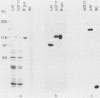
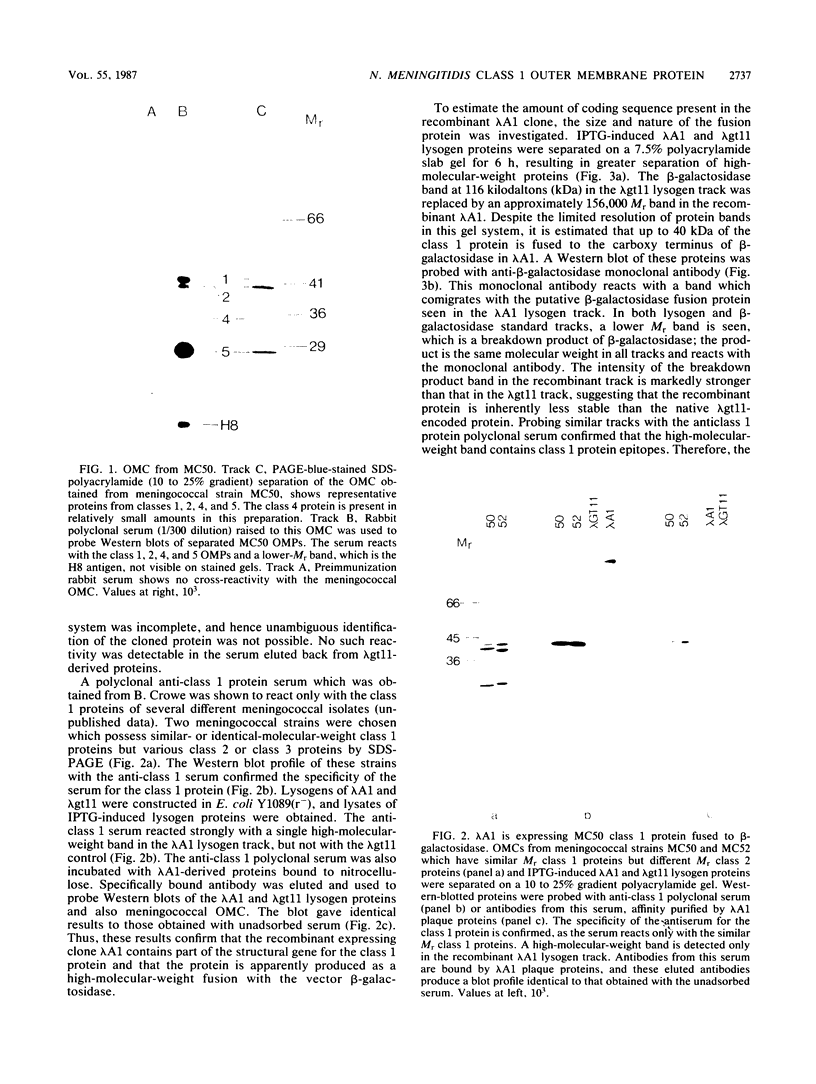
A genomic library of meningococcal DNA from a clinical isolate of Neisseria meningitidis was constructed in the expression vector lambda gt11. Outer membrane complex was prepared from the same strain and used to immunize rabbits to raise polyclonal anti-outer membrane complex serum. The amplified library was probed with this polyclonal serum, and seven expressing recombinants were isolated; further investigations indicated these to be identical. The expressed meningococcal gene in these recombinants was fused to vector beta-galactosidase and shown to encode epitopes present on the 42-kilodalton class 1 outer membrane protein. Estimation of the size of the recombinant fusion protein suggests that up to 40 kilodaltons of protein-coding sequence is present. The lambda gt11 recombinant contains a 3.4-kilobase DNA insert, which has been recloned into a plasmid and characterized by restriction endonuclease analysis. A restriction fragment from the insert, representing the protein-coding region hybridizes to a single 2.2-kilobase XbaI fragment from the homologous strain and to similar-sized XbaI fragments in other strains of meningococci, expressing antigenically distinct class 1 proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia F. F., Inouye S., Inouye M. A 26-base-pair repetitive sequence specific for Neisseria gonorrhoeae and Neisseria meningitidis genomic DNA. J Bacteriol. 1986 Sep;167(3):1009–1015. doi: 10.1128/jb.167.3.1009-1015.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Zollinger W. D., Poolman J. T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985 Jul-Aug;7(4):504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Blake M. S., Koomey J. M., Seiff M., Derman A. Cloning of the structural genes of three H8 antigens and of protein III of Neisseria gonorrhoeae. J Exp Med. 1986 Sep 1;164(3):868–881. doi: 10.1084/jem.164.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. A., Gwaltney J. M., Jr, Hendley J. O., Farquhar J. D., Samuelson J. S. Clinical and serological evaluation of a meningococcal polysaccharide vaccine groups A, C, Y, and W135. Proc Soc Exp Biol Med. 1982 Jan;169(1):54–57. doi: 10.3181/00379727-169-41306. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Sarna S., Mäkelä P. H. Serum antibodies to capsular polysaccharide vaccine of group A Neissera meningitidis followed for three years in infants and children. J Infect Dis. 1980 Dec;142(6):861–868. doi: 10.1093/infdis/142.6.861. [DOI] [PubMed] [Google Scholar]
- Lynch E. C., Blake M. S., Gotschlich E. C., Mauro A. Studies of Porins: Spontaneously Transferred from Whole Cells and Reconstituted from Purified Proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys J. 1984 Jan;45(1):104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., de Marie S., Zanen H. C. Variability of low-molecular-weight, heat-modifiable outer membrane proteins of Neisseria meningitidis. Infect Immun. 1980 Dec;30(3):642–648. doi: 10.1128/iai.30.3.642-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Newport G., Agabian N. Molecular cloning and expression of Chlamydia trachomatis major outer membrane protein antigens in Escherichia coli. Infect Immun. 1985 Mar;47(3):713–718. doi: 10.1128/iai.47.3.713-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley C. R., Heckels J. E. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J Gen Microbiol. 1986 Sep;132(9):2483–2490. doi: 10.1099/00221287-132-9-2483. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Mocca L. F. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981 Apr;146(1):69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Moran E. E., Connelly H., Mandrell R. E., Brandt B. Monoclonal antibodies to serotype 2 and serotype 15 outer membrane proteins of Neisseria meningitidis and their use in serotyping. Infect Immun. 1984 Oct;46(1):260–266. doi: 10.1128/iai.46.1.260-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]