Abstract
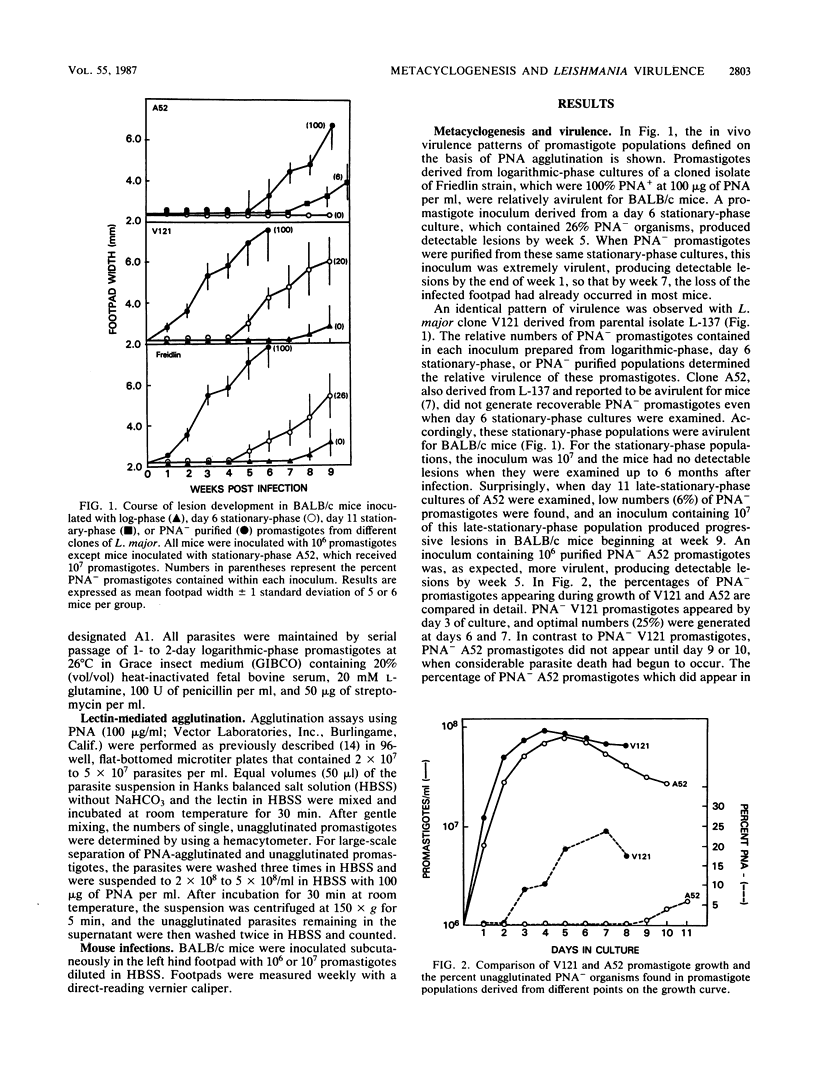
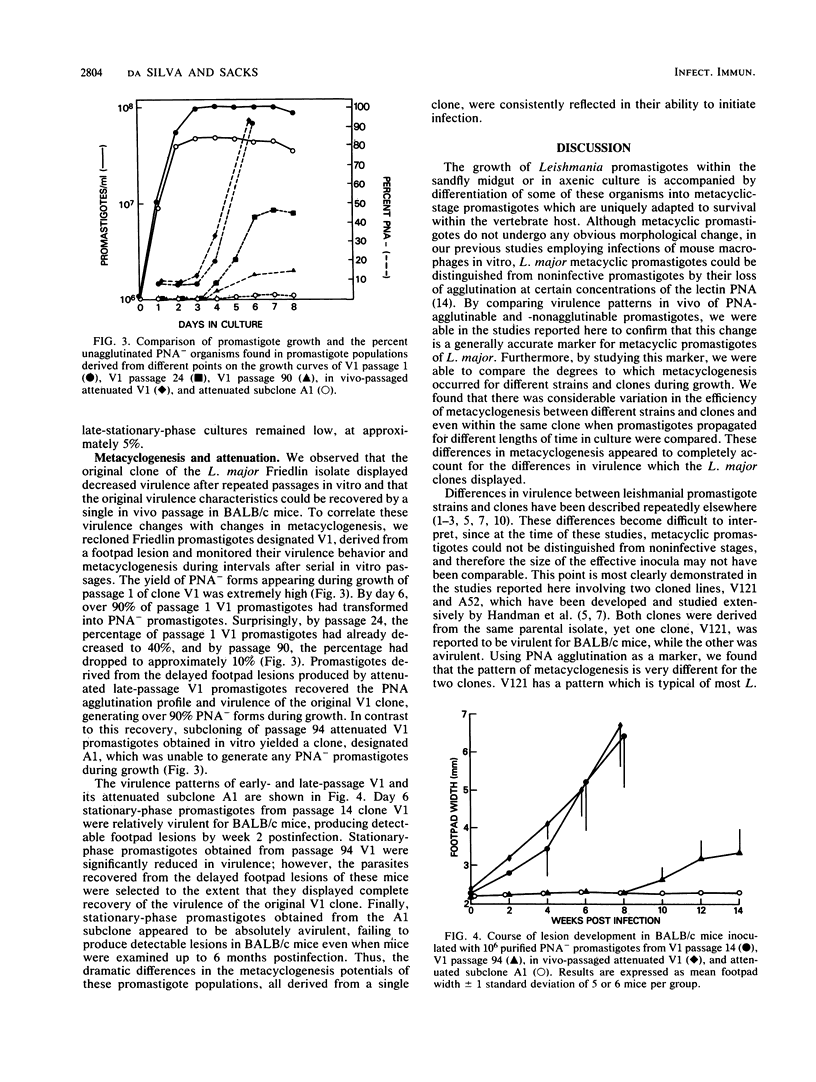
The in vivo virulence patterns of promastigote populations defined on the basis of agglutination by the lectin peanut agglutinin (PNA) were studied for various cloned lines of Leishmania major. Promastigotes derived from logarithmic-phase cultures, which were routinely 100% agglutinated at 100 micrograms of PNA per ml, were relatively avirulent for BALB/c mice. The relative virulence of stationary-phase promastigotes appeared to be attributable to the proportion of nonagglutinable (PNA-) promastigotes contained within these populations. Purification of PNA- organisms from stationary cultures provided for each clone the most virulent inoculum, supporting the view that this change in lectin binding accurately reflects the development of infective metacyclic stage promastigotes. By studying this marker, we found that there was considerable variation in the degree to which different strains and clones underwent metacyclogenesis during growth. Examination of a reportedly avirulent L. major clone revealed that metacyclogenesis was unusually delayed and inefficient for this clone, but that those PNA- promastigotes which could be recovered from late-stationary-phase cultures were virulent for BALB/c mice. The loss of virulence associated with frequent subculture could also be attributed to a drastic diminution in metacyclogenesis potential over time. A clone which yielded over 90% PNA- promastigotes during growth within passage 1 generated fewer than 10% PNA- promastigotes during growth by passage 94. Subcloning of late-passage attenuated promastigotes yielded a clone for which no PNA- promastigotes could be generated during growth, and an infective population could not be derived from this clone. Thus, metacyclogenesis does not appear to be stable for even cloned lines of Leishmania promastigotes, and virulence comparisons between different strains and clones can be meaningfully made only if the metacyclic populations contained within the respective inocula are determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayesta C., Argüello C., Hernández A. G. Leishmania braziliensis: cell surface differences in promastigotes of pathogenic and nonpathogenic strains. Exp Parasitol. 1985 Apr;59(2):185–191. doi: 10.1016/0014-4894(85)90071-2. [DOI] [PubMed] [Google Scholar]
- Dawidowicz K., Hernandez A. G., Infante R. B., Convit J. The surface membrane of Leishmania. I. The effects of lectins on different stages of Leishmania braziliensis. J Parasitol. 1975 Oct;61(5):950–953. [PubMed] [Google Scholar]
- Ebrahimzadeh A., Jones T. C. A comparative study of different Leishmania tropica isolates from Iran: correlation between infectivity and cytochemical properties. Am J Trop Med Hyg. 1983 Jul;32(4):694–702. doi: 10.4269/ajtmh.1983.32.694. [DOI] [PubMed] [Google Scholar]
- Giannini M. S. Effects of promastigote growth phase, frequency of subculture, and host age on promastigote-initiated infections with Leishmania donovani in the golden hamster. J Protozool. 1974 Oct;21(4):521–527. doi: 10.1111/j.1550-7408.1974.tb03692.x. [DOI] [PubMed] [Google Scholar]
- Greenblatt C. L., Handman E., Mitchell G. F., Battye F. L., Schnur L. F., Snary D. Phenotypic diversity of cloned lines of Leishmania major promastigotes. Z Parasitenkd. 1985;71(2):141–157. doi: 10.1007/BF00926265. [DOI] [PubMed] [Google Scholar]
- Grimaldi G., Jr, Momen H., Soares M. J., Moriearty P. L. Enzyme variation and difference in infectivity within a single strain of Leishmania mexicana mexicana. Int J Parasitol. 1982 Jun;12(2-3):185–189. doi: 10.1016/0020-7519(82)90015-7. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Katakura K., Kobayashi A. Enhancement of infectivity of Leishmania donovani promastigotes by serial mouse passages. J Parasitol. 1985 Jun;71(3):393–394. [PubMed] [Google Scholar]
- Keithly J. S. Infectivity of Leishmania donovani amastigotes and promastigotes for golden hamsters. J Protozool. 1976 May;23(2):244–245. doi: 10.1111/j.1550-7408.1976.tb03763.x. [DOI] [PubMed] [Google Scholar]
- NEAL R. A. CHEMOTHERAPY OF CUTANEOUS LEISHMANIASIS: LEISHMANIA TROPICA INFECTIONS IN MICE. Ann Trop Med Parasitol. 1964 Dec;58:420–430. doi: 10.1080/00034983.1964.11686264. [DOI] [PubMed] [Google Scholar]
- Rizvi F. S., Afchain D., Sherlock I., Sadigursky M., Capron A., Santoro F. Infectivity of Leishmania promastigotes is associated with surface antigenic expression. Immunol Lett. 1985;11(5-6):317–323. doi: 10.1016/0165-2478(85)90114-2. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Hieny S., Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985 Jul;135(1):564–569. [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am J Trop Med Hyg. 1985 May;34(3):456–459. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Wonde T., Honigberg B. M. Morphology and infectivity of Leishmania donovani cultivated in nonliving media at elevated temperatures. Am J Trop Med Hyg. 1971 Nov;20(6):828–838. doi: 10.4269/ajtmh.1971.20.828. [DOI] [PubMed] [Google Scholar]
- Wozencraft A. O., Blackwell J. M. Increased infectivity of stationary-phase promastigotes of Leishmania donovani: correlation with enhanced C3 binding capacity and CR3-mediated attachment to host macrophages. Immunology. 1987 Apr;60(4):559–563. [PMC free article] [PubMed] [Google Scholar]



