Abstract
ArsR (or ArsR/SmtB) family metalloregulatory homodimeric repressors collectively respond to a wide range of metal ion inducers in regulating homeostasis and resistance of essential and nonessential metal ions in bacteria. BxmR from the cyanobacterium Osciliatoria brevis is the first characterized ArsR protein that senses both CuI/AgI and divalent metals ZnII/CdII in cells by regulating the expression of a P-type ATPase efflux pump (Bxa1) and an intracellular metallothionein (BmtA). We show here that both pairs of predicted α3N and α5 sites bind metal ions, but with distinct physicochemical and functional metal specificities. Inactivation of the thiophilic α3N site via mutation (C77S) abolishes regulation by both CdII and CuI, while ZnII remains a potent allosteric negative effector of operator/promoter binding (ΔGc≥+3.2 kcal mol−1). In contrast, α5 site mutant retains regulation by all four metal ions, albeit with a smaller coupling free energy (ΔGc≈+1.7 (±0.1) kcal mol−1). Unlike the other metals ions, the BxmR dimer binds four mol•equiv CuI to form an α3N binuclear CuI2S4 cluster by x-ray absorption spectroscopy. BxmR is thus distinguishable from other closely-related ArsR family sensors, in having evolved a metalloregulatory α3N site that can adopt an expanded range of coordination chemistries, while maintaining redundancy in the response to ZnII. The evolutionary implications of these findings for the ArsR metal sensor family are discussed.
All cells must obtain their quota of biologically required transition metal ions for use as cofactors in metalloenzymes or for structural or regulatory roles through specific membrane-associated transporters or uptake systems (9). However, all metal ions are toxic in excess, and the intracellular availability of each is tightly controlled in such a way that metal homeostasis allows organisms to rapidly respond to changes in their microenvironments (10, 11). Likewise, metal ions that play no biological role, e.g., heavy metal pollutants and other environmental contaminants, must either be detoxified via biotransformation or efflux from the cytosol (12, 13).
This adaptive response is mediated by gene regulatory proteins, coined metalloregulatory proteins (10), or metal sensor (14) proteins. These specialized proteins have evolved metal coordination sites that "sense" specific metals ion(s) in the cytosol by forming specific coordination complexes. This, in turn, functions to activate or inhibit DNA binding to operator sites(s) in the promoter thereby regulating the expression of genes. These genes include transporters, intracellular chelators and detoxification enzymes, that mediate metal homeostasis or resistance in what is thought to be a selective adaptive response.
There are currently seven major families of metal-sensing transcriptional regulators that have thus far been structurally characterized in prokaryotes (see (14) for a review). These seven families span the detection of the six primary biologically essential first row transition elements MnII, FeII, CoII, NiII, CuI and ZnII, as well as nonbiological heavy metals AgI/AuI and CdII/HgII, respectively, found in the environment. In addition, ArsR1 or ArsR/SmtB (6) and MerR (15) family sensors have been identified that detect PbII, as well as AsIII/SbIII and BiIII. Sensor families are typically named for the founding member(s) that gave rise to the family (14). Of interest here is the ArsR/SmtB family, named for E. coli R774 ArsR, which regulates expression of the ars operon in response to trivalent AsIII and SbIII and their oxyanions and organoarsenicals (16) and the ZnII sensor Synechococcus SmtB (17), which regulates expression of the smt (Synechococcus metallothionein) operon largely as a result of ZnII toxicity.
ArsR family sensors (COG0640) constitute a very large class of bacterial transcriptional regulators. A recent report identifies over 500 unique sequences that could be divided into eight major groups on the basis of global sequence similarity (7). A least four structurally distinct, subunit-bridging metal sensing sites have now been identified and characterized in ArsR family sensors, a remarkable collective feature that appears to distinguish the ArsR family from all other metalloregulatory protein classes (4, 14). In other metal sensor families, a single regulatory metal site often acquires distinct metal specificities by more subtle structural alterations in the first coordination shell (14, 18, 19), a feature that also characterizes ArsR sensors that harbor a single subtype of metal site, e.g., α5/α5C (see below) (20).
In ArsR family proteins, metal sites are named for the secondary structural elements that are known or thought to provide ligand donor atoms to the individual metal ions (20, 21). These sites have been designated α3N/α3 (21) (or metal site 1 in S. aureus pI258 CadC (1)), α4C (22), α5/α5C (3, 21, 23) (site 2 in S. aureus pI258 CadC), and most recently α5-3 (7).1 Further evidence for an even greater structural diversity of sensing sites in ArsR family proteins comes from the recent characterization of two AsIII-sensing ArsRs shown to harbor tris-thiolato AsIII binding sites distinct from the S3 α3 site originally characterized in E. coli R773 ArsR (4, 5). Similarly, our physical characterization of a predicted CdII/PbII-sensing CmtR from Streptomyces coelicoler (SCO0875) reveals a second S3 CdII/PbII binding site involving a pair of adjacent Cys residues at the extreme C-terminus (Y. Wang and D. Giedroc, unpublished observations) in addition to the canonical α4C CdII/PbII site structurally characterized (24, 25).
While the metal selectivity of individual metal site subtypes is rather well understood and sometimes predictable on the basis of the amino acid sequence (20, 26), approximately half of the projected ArsR family members do not conform to any of these four metal-binding motifs. For example, one poorly characterized subgroup contains a conserved Met and two conserved Cys, the latter derived from the projected α2 and α5 helices that are predicted to be quite close in the structure but may not bind metal ions directly (7, 27). These include Paracoccus pantotrophus SoxR, the thiosulfate-induced sulfur-oxidizing (sox) operon regulator (28), V. cholerae HyU (7) and Xylella fastidiosa BigR, a regulator implicated biofilm formation (29). Thus, it remains a considerable challenge to unambiguously identify the mode of induction of ArsR sensors that do not precisely conform to one of the four known structural classes on the basis of amino acid sequence alone. In addition, since some ArsR family sensors contain two metal sites, e.g., α3N and α5 (6, 30), a further complication is that one site might can be metalloregulatory while the other site may bind metal ions but in a way that does not mediate allosteric regulation of DNA binding, and is thus nonfunctional (30, 31).
Initial biological studies established that an ArsR family repressor, termed BxmR1, regulated the expression of genes encoding both a heavy metal transporting ATPase, Bxa11, as well as an intracellular metallothionein, BmtA1, in the freshwater cyanobacterium Oscillatoria brevis (32, 33). Oscillatoria spp. produce geosmin and 2-methylisoborneaol which give drinking water a musty or earthy odor and has a major negative economic impact on the catfish aquaculture industry due to absorption in the flesh of the fish (34). Copper sulfate has been extensively used in water purification systems as a copper algaecide (34), and it was subsequently demonstrated that bxa1 expression was inducible by both monovalent (CuI/AgI) and divalent (ZnII/CdII) ions in O. brevis, a novel property (33). The thiol oxidant diamide is also an inducer of bmtA expression (35). In vitro DNA binding and coupled transcription-translation experiments suggested that metal and diamide stress-induced disassembly of BxmR-operator/promoter complexes was directly responsible for regulation (35, 36).
In this report, we present a comprehensive characterization of the metal binding properties and metalloregulation of bxa1 operator/promoter binding by BxmR (33). We show that both pairs of predicted α3N and α5 sites bind metal ions, but with distinct physicochemical and functional metal specificities. BxmR is thus distinguished from other closely-related ArsR family sensors, in having evolved a metalloregulatory α3N site that can adopt an expanded range of coordination chemistries to include CuI/AgI, while maintaining redundancy in the response to ZnII. These studies provide a glimpse as to how an ArsR family metal sensor with a relaxed metal specificity profile could have evolved to effect resistance against an expanded range of heavy metal ions.
EXPERIMENTAL PROCEDURES
Construction of E. coli overexpression plasmids for BxmRs and protein purification
The wild-type bxmR-coding region was amplified by PCR from O. brevis genomic DNA and cloned into pET3a (Novagen) between NdeI and EcoRI restriction sites to construct pET3a-BxmR. In order to obtain overexpression clones for α3NΔ and α5Δ BxmRs, a PCR-based quick-change mutagenesis method was employed to replace Cys77 to Ser in the α3N site, or 132HLDEE136 to 132NLTYA136 (H132N/D134T/E135Y/E136A) in the α5 site, using pET3a-BxmR as a template to create pET3a-α3NΔ BxmR and pET3a-α5Δ BxmR, respectively. C31S BxmR was prepared in exactly the same way. The integrity of all plasmid constructions was confirmed by complete DNA sequencing.
The resultant constructs were transformed into E. coli BL21(DE3) and grown in LB to mid-log phase, after which time 0.4 mM IPTG was added and cells allowed to grow for an additional 2.5 h. Freshly harvested cells were pelleted by low-speed centrifugations and suspended in 100 mL Buffer A (25 mM MES, 5 mM DTT, 1 mM EDTA, pH 6.0) and lysed by sonication. The cellular lysate was centrifuged and the supernatant pooled and subjected to precipitation by addition of polyethylenimine (PEI) to 0.15% (v/v) at pH 6.0. The PEI supernatant and precipitated fractions were collected separately. The PEI pellet was resuspended in Buffer A containing 0.50 M NaCl to extract bound proteins. Protein fractions derived from the NaCl-washed PEI pellet were precipitated by the addition of solid (NH4)2SO4 to 50% saturation. Each ammonium sulfate pellet was gently resuspended in 100 mL Buffer A with 0.05 M NaCl and separately subjected to SP Fast Flow chromatography (20 mL bed volume) on an Äkta-10 purifier, with elution achieved with a linear NaCl gradient (0.05-0.75 M) in Buffer A. BxmR-containing fractions eluted at approximately 0.15 M NaCl and 0.35 M NaCl and were pooled separately. The resultant protein fractions were further purified by Superdex 75 size-exclusion chromatography, and anion-exchange HPLC using a procedure similar to that described previously (8). Individual fractions containing highly purified BxmR were pooled, concentrated and dialyzed against 3 L of Buffer B (10 mM HEPES, 0.40 M NaCl, pH 7.0) in an anaerobic Vacuum Atmospheres glovebox. The purity of the final products was estimated by visualization of Coomassie-stained 18% Tris-glycine SDS-PAGE gels to be ≥95%. The concentrations of all BxmR stock solutions were determined by quantitative amino acid analysis carried out by the Texas A&M University Protein Chemistry Laboratory. The number of reduced thiols in wild-type BxmR was determined by anaerobic DTNB colorimetric assay to be 4.1 (4.0 expected) and 2.8 (3 expected) in the WT and C31S BxmRs (8). The possible presence of copper in apo-BxmR was determined using a Perkin-Elmer AAnalyst 700 atomic absorption spectrophotometer operating in the flame mode using hollow cathode lamps specific for each metal (37). The purification of α3NΔ (C77S) and α5Δ BxmRs were purified in essentially the same fashion and characterized as indicated.
Copper Binding Experiments
All metal binding experiments were carried out anaerobically at ambient temperature (≈22 °C) using either a Hewlett-Packard Model 8452A Spectrophotometer (for UV-Vis electronic absorption spectra) or at 25 (±0.1) ºC with an ISS PC1 photon counting spectrofluorometer (for fluorescence and luminescence titrations, respectively) essentially as described previously (37, 38). Solution conditions were 10 mM MES, 0.1 M NaCl, pH 6.3 unless otherwise indicated. 10.5 and 11.2 µM wild-type and C31S BxmR monomer, respectively, was used for the UV-Vis absorption experiments, while 10.0 µM BxmR monomer was used for the luminescence experiments (λex=300 nm with the emission spectrum scanned from 500-750 nm). For tyrosine fluorescence measurements, 5.0 μM BxmR monomer in Buffer B (10 mM HEPES, 0.40 M NaCl, pH 7.0) was titrated with 2-5 μL aliquots of either a 400 μM CuI stock in Buffer B or mock-titrated with Buffer B (λex=280 nm; λem=305 nm). At each ith addition, the emission intensity was recorded and corrected for dilution, photobleaching (from the mock-titrated sample) and the inner filter effect exactly as described previously (21).
Other Metal Binding Experiments
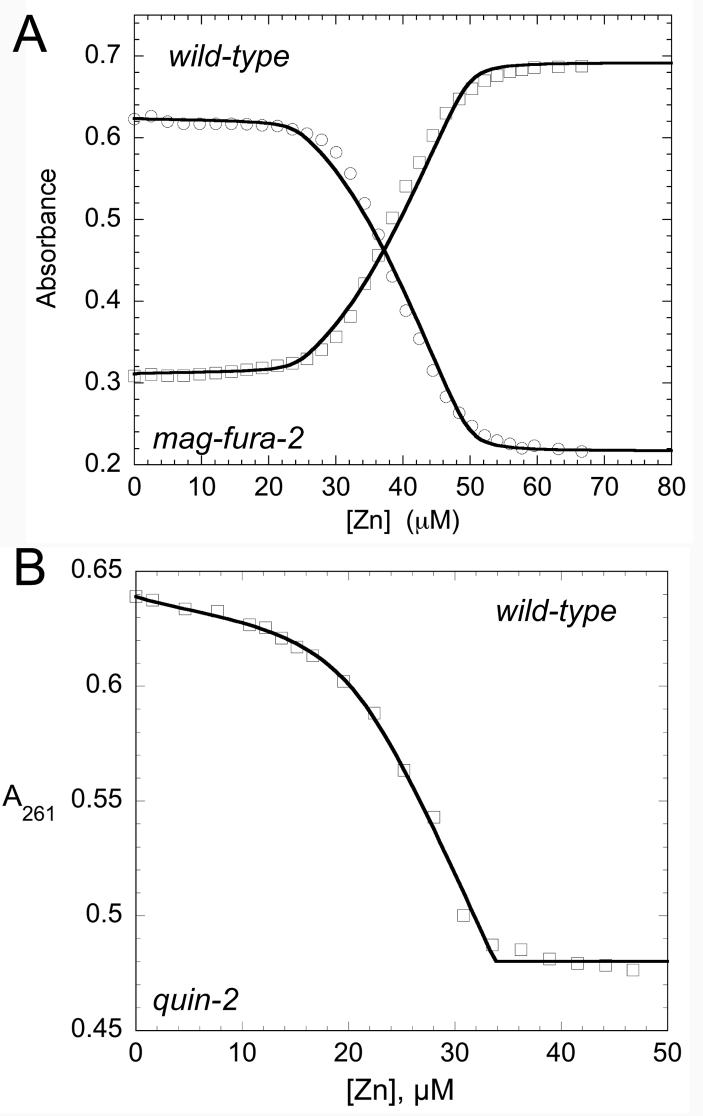
ZnII binding stoichiometries and affinities of wild-type, α3NΔ and α5Δ BxmRs were determined using chelator competition assays with either mag-fura-21(KZn=5.0 × 107 M−1 at pH 7.0) (39) or quin-21 (KZn=2.7 × 1011 M−1 at pH 7.0, 25 ºC) (40) using absorption spectroscopy essentially as described (23, 41). These data were fit using a competitive binding model with DynaFit (42) to determine step-wise zinc binding affinities, KZni. For mag-fura-2 experiments, 25 µM BxmR dimer (12.5 µM monomer) was mixed with 16.0 µM mag-fura-2 and titrated with a known stock solution of ZnII; for quin-2 experiments, 20 µM BxmR dimer was mixed with 15 µM quin-2 and titrated with ZnII (see Table 1). CoII and CdII binding experiments were carried out essentially as described previously (30, 43) with the solution conditions given in the figure legends. The metal concentrations of all metal titrants used in individual experiments was quantified by atomic absorption spectroscopy as described (8).
Table 1.
Step-wise zinc binding affinities, KZni, derived from mag-fura-2 and quin-2 chelator competition experimentsa
| BxmR protein | chelator | KZn1 (M−1) | KZn2 (M−1) | KZn3 (M−1) | KZn4 (M−1) |
|---|---|---|---|---|---|
| wild-type | mag-fura-2 | ≥109 a | ≥109 | 2.2 × 107 | n.d.b |
| quin-2 | 9.4 × 1012 | 1.1 × 1012 | n.d.c | n.d.c | |
| α3NΔ | mag-fura-2 | ≥109 a | 6.0 × 107 | – | – |
| quin-2 | 6.4 × 1012 | n.d.c | – | – | |
| α5Δ | mag-fura-2 | ≥109 a | 3.0 × 106 | – | – |
| quin-2 | 4.6 × 1010 | n.d.c | – | – |
From nonlinear least-squares fits to data like those shown in Fig. 2 to a model assuming four (wild-type) or two (α3NΔ and α5Δ) stepwise binding events to a nondissociable dimer, each characterized by an affinity of KZni.
n.d., binding not detected, or KZni≤105 M−1 (a lower limit for mag-fura-2).
n.d., binding not detected, or KZni≤109 M−1 (a lower limit for quin-2).
Fluorescence Anisotropy-based DNA Binding Experiments
These experiments were carried out essentially as described previously (23, 41) using an ISS PC1 spectrofluorometer fitted with polarizers in the L format. 52-base pair oligonucleotide duplexes were prepared by mixing one unlabeled “bottom” strand at a slight excess relative to a 5′-fluorescein labeled “top” strand, annealed by heating to 95 ºC and slow-cooling at room temperature. Duplexes corresponding to the bxa1 and bxmR operator-promoter (O/P) regions contained a 26-base pair TGAA-containing inverted repeat (see ref. (36) for sequences) centered in the 52-base pair duplex. Titrations were carried out with 10 nM duplex in Buffer B (10 mM Hepes, 0.4 M NaCl, pH 7.0, 1.0 mM dithiothreitol) at 25 (±0.1) ºC with either apo-BxmRs or BxmRs preincubated with the indicated amount of a metal ion, e.g., CuI, AgI, ZnII or CdII. Experimental binding curves were fit using DynaFit (42) to a binding model that assumes that two dimers bind to the DNA with identical binding affinities (K1=K2) (36), linked to a monomer-dimer equilibrium constant, Kdim, of 5.0 × 106 M−1 (44) The characteristic anisotropy of the 2:1 dimer:DNA species, r(P2)2D, was optimized during the fit, with the r(P2) D value fixed to exactly halfway between r(P2)2D and rD. In this work, the allosteric coupling free energies, ΔGc, was operationally defined as the logarithmic ratio K1metal/K1apo according to ΔGc = −RTln(K1metal/K1apo) with T=298 K.
RESULTS
Sequence alignment of selected ArsR regulators
O. brevis BxmR contains complete predicted ligand donor sets for both the interprotomer α3N and α5 metal sites previously characterized in other ArsR family sensors (6, 21). The α3N site is thiophilic site with a diagnostic 75CVC77 metal-binding sequence (30), and a number of potential donor residues in the N-terminal region, including by Cys23, His27 and Cys31 (36); the multiple sequence alignment of α3N sensors reveals that Cys31 in BxmR is unique (Figure 1). Cys23, Cys31, Cys75 and Cys77 are the only Cys in BxmR. The α5 site is a well characterized tetrahedral ZnII-sensing site composed of carboxylate and imidazole ligands (3, 23); in BxmR, these are Asp119, His121, His132′ and Glu135′ with the His corresponding to His132 the key allosteric residue in other ZnII sensors CzrA and SmtB (23). α3NΔ BxmR corresponds to C77S BxmR; an analogous non-metal liganding substitution abolishes CdII binding and sensing by S. aureus CadC (30, 45) and CdII/ZnII sensing by Anabaena AztR (8). α5Δ BxmR is a quadruple mutant (H132N/D134T/E135Y/E136A) that changes two of the four α5 site ligands, His132 and Glu135 to non-metal liganding residues found in Anabaena AztR (8); these substitutions abolish ZnII/CoII binding to this site in AztR.
Figure 1.
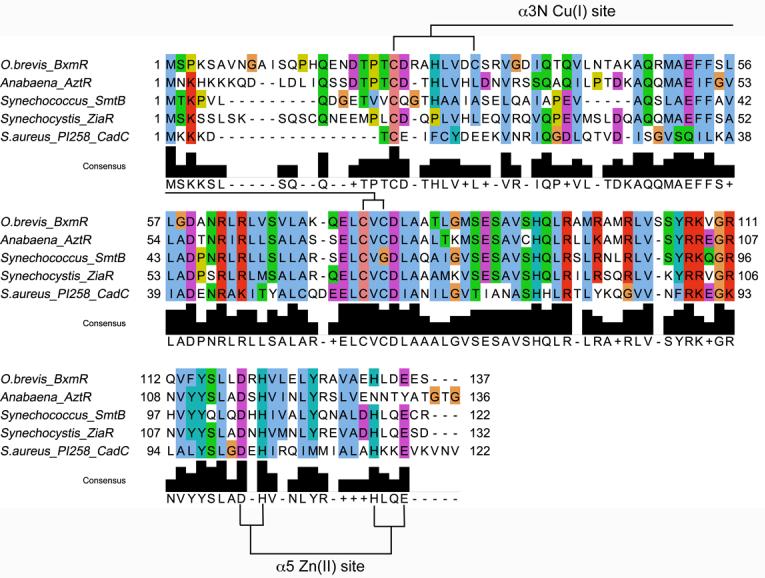
Multiple sequence alignment of BxmR with other ArsR/SmtB family divalent metal ion-sensing regulatory transcriptional repressors (6, 8, 21, 30, 52). The proposed primary CuI binding ligands in α3N site and the α5 site for ZnII are indicated for BxmR (this work).
ZnII binding by wild-type, α3NΔ and α5Δ BxmRs
Like other previously characterized ArsR sensors (20, 44), BxmR is a stable dimer in solution as revealed by dynamic light scattering and gel filtration chromatography (data not shown). As a result, wild-type BxmR is predicted to contain four subunit-bridging metal binding sites per dimer (or two per protomer), two α3N and two α5 sites. We used standard dye competition experiments with two different chelators of widely different affinities, mag-fura-2 (KZn=5.0 × 107 M−1) and quin-2 (KZn=2.7 × 1011 M−1), in order to quantify the stoiochiometry and affinity of the ZnII binding sites in wild-type, α3NΔ and α5Δ BxmRs (3, 21). Representative mag-fura-2 and quin-2 competition titrations carried out with wild-type BxmR are shown in Fig. 2, with parameter values obtained from a simple step-wise binding model shown in Table 1 for all BxmR species investigated here. Wild-type BxmR binds three ZnII ions with an affinity that is either comparable to, or far greater than, that of mag-fura-2, with the fourth site transparent in this assay (KZn≤105 M−1) (Table 1). Using the higher affinity competitor dye, quin-2, reveals just two high affinity sites, with KZn values of ≈1013 and 1012 M−1; this result is consistent with the mag-fura-2 titrations. Carrying out the same experiments with α3NΔ and α5Δ BxmRs, where one pair of symmetry-related metal sites is inactivated in each case, clearly shows that each mutant binds two metal ions, one with very high affinity (KZn>109 M−1), measurable with quin-2 (Table 1), and one with an affinity comparable to or less than that of mag-fura-2 (KZn≤107 M−1) (see Supplementary Figure S1; Table 1). These binding parameters are diagnostic for strong negative homotropic cooperativity of ZnII binding to each pair of metal sites, previously documented in the α3N sensor CadC (44) and structurally validated in the α5 sensors CzrA (3, 41) and SmtB (21).
Figure 2.
ZnII binding by wild-type BxmR homodimer. (A) Titration of a mixture of 25 µM (monomer) BxmR and 16 µM mag-fura-2 with ZnII with the absorption monitored simultaneously at 366 nm (μ) and 325 nm (open squares). (B) Titration of a mixture of 20 µM (monomer) BxmR and 15 µM quin-2 with ZnII, with the absorption monitored at 261 nm. In both cases, the solid lines represent a fit to a multiple site binding model, with the parameters compiled in Table 1. Analogous titrations were carried out with α3NΔ and α5Δ BxmRs under the same conditions (see Supplementary Figure S1) with these parameters compiled in Table 1.
Taking all of the data together, the simplest interpretation is that ZnII fills the metal binding sites on the wild-type BxmR homodimer in the following order: α5 H α3N ⇒ α5′ H α3N′. Thus, one of the α5 sites and one of the α3N sites possesses very high intrinsic zinc affinities KZn≥1012 M−1 or nearly equivalent to that found previously in the ZnII sensor CzrA (3, 41); as a result, the dominant ZnII complex that would persist over a wide range of [ZnII]free is the BxmR α51/α3N1 homodimer. Further evidence for this formation of an α51/α3N1 metallated homodimer comes from ZnII binding isotherms monitored by a change in the steady-state Tyr fluorescence of 5.0 µM BxmRs (Figure 3). BxmR contains the same three Tyr residues found in the known structure of SmtB (2, 3), two in each strand of the β−wing, and one in the α5 helix (Fig. 1). As previously found for SmtB, ZnII binding to the α5 site in α3NΔ BxmR gives rise to small but measurable enhancement or increase in the Tyr fluorescence intensity (21). In contrast, binding to the α3N site in α5Δ BxmR gives rise to the large quenching of the Tyr fluorescence not observed in SmtB; this spectral change is likely reporting on changes in the β-wing conformation due to α3N site binding. Both α3NΔ and α5Δ BxmR ZnII binding isotherms reach maximum (or minimum) F/Fo values at ≈0.5:1 ZnII to monomer consistent with the idea that half the sites drive the conformational change in the dimer as found previously for the α5 site in CzrA (41). Further, the conformational changes may well be distinct since the fluorescence yields of Tyr are different in each case. Strikingly, the addition of ZnII to wild-type BxmR gives rise to a binding curve that is best described as a linear combination of ZnII bound to either the α5 or α3N site, i.e., added ZnII partitions between the two sites, which then reaches a minimum F/Fo value at ≈1:1 ZnII to monomer (2 per dimer); residual binding of the third ZnII to the second α3N site results in a small upward curvature of the F/Fo values (Figure 3).
Figure 3.
Anaerobic titration of 5.0 µM wild-type BxmR monomer (2.5 µM dimer) (μ), α3NΔ (λ) and α5Δ (υ) BxmRs with ZnII as monitored by change in the Tyr fluorescence intensity, Fi/Fo. The solid lines through the data correspond to a nonlinear least squares fit to model that invokes sequential binding of ZnII to a single high affinity site (for α3NΔ and α5Δ BxmRs) or two high affinity sites (for wild-type BxmR) each with a characteristic Fi/Fo signature. After filling these high affinity zinc binding sites (one α5 site with an Fi/Fo>1; one α3N site with an Fi/Fo <1), Zn(II) binds to each of the remaining low affinity α5′ and α′ sites on the dimer. Fitted parameters are as follows, with KZn1>1010 M−1 (see Fig. 2A; Table 1): α3NΔ BxmR: Fi/Fo values, 1.045 (±0.001), 1.03 (fixed) for the high affinity and low affinity α5 Zn(II) sites, respectively; α5Δ BxmR: Fi/Fo values, 0.753 (±0.002), 0.915 (±0.011) for the high affinity and low affinity α3N Zn(II) sites, respectively; wild-type BxmR, Fi/Fo constrained to the above values for first three binding steps, with Fi/Fo for filling the final binding site, 0.80 (±0.03).
CoII binding by wild-type BxmR
While CoII is not an inducer of the bxa1 or bmtA genes (36), CoII is often used as a structural surrogate for ZnII to obtain insight into coordination geometry and nature of the ligand donor atoms. Interestingly, the sequence of metal binding steps for ZnII is in contrast to that observed for CoII, which largely fills both α5 sites, followed by both α3N sites in wild-type BxmR, an interpretation facilitated by the distinct spectral features of the CoII complexes of the α5 and α3N sites (8, 21, 30) (Supplementary Figure S2). Both sites adopt tetrahedral or distorted tetrahedral coordination geometries consistent with S3(N/O) α3N and N2O2 α5 ligand donor sets (8, 21). As expected, addition of stoichiometric ZnII effectively bleaches the CoII-α5 visible absorption spectrum in α3NΔ BxmR, consistent with KZnα5>>KCoα5 as expected for tetrahedral symmetry (43).
CdII binding by wild-type, C31S, α3NΔ and α5Δ BxmRs
Representative CdII binding curves are shown for wild-type and C31S BxmRs as monitored by Cys S−⇒ CdII ligand-to-metal (or metal-to-ligand) charge transfer absorption at 235 nm (Supplementary Figure S3). The binding curves in both cases are essentially stoichiometric and reveal a single CdII binding site per monomer (2 per dimer) characterized by a molar absorptivity at 235 nm of ≈20,000 M−1 cm−1 for wild-type BxmR, most consistent with a tetrahedral S4 complex (30). This binding curve is reporting on filling of the α3N sites with CdII, with any binding to the carboxy-terminal α5 sites not detected in this assay since this site contains no thiolate ligands. CdII does bind here, but with a lower affinity than CoII since a 4-fold molar excess of CdII retains significant CoII absorption deriving from the α5 site in α3NΔ BxmR (data not shown). Since we observe a lower molar absorptivity with the C31S BxmR (ε235=16,000 MCd−1 cm−1), the simplest interpretation is that CdII coordinates to all four Cys in BxmR (Cys23, Cys31, Cys75, Cys77) to create a distorted tetrahedral S4 site. As expected from prior studies of CadC (30, 46), CdII binds to the mutant α3N sites in α3NΔ BxmR, but with a lower affinity and lower molar absorptivity (ε235=7000 M−1 cm−1); this is indicative of a significantly perturbed first coordination shell (data not shown).
CuI binding by wild-type, C31S and α5Δ BxmRs
Previous studies have established expression of both the bxa1 and bmtA genes is responsive to Cu/Ag and that the addition of reduced CuI or AgI to preformed BxmR-bxa1 and BxmR-bxmR/bmtA operator-promoter fragments in vitro resulted in dissociation of these complexes (36). Although these results are consistent with a functionally important and direct interaction of the CuI/AgI with BxmR, they provided no insight into the nature of the coordination complexes formed by CuI/AgI.
We measured CuI binding to apo-forms of wild-type and C31S BxmR anerobically using two different spectroscopic techniques. These include UV-visible absorption which reports on Cys S−⇒ CuI ligand-to-metal charge transfer absorption (LMCTs), i.e., formation of cysteine-copper coordination bonds, and luminescence in the visible region (λex=300 nm; λem=580 nm). The results of these experiments are shown for wild-type and C31S BxmRs in Fig. 4A, B and Fig. 4C, D, respectively. CuI binding isotherms monitored in the UV region are consistent with a limiting stoichiometry of two CuI/monomer or four per dimer in both cases (Fig. 4A, C). While the C31S CuI binding curve is essentially stoichiometric under these conditions (pH 6.3, 25 ºC), the binding curve for wild-type BxmR can be fit to two step-wise equilibrium binding affinities, KCu1 and KCu2 of 3.6 × 107 M−1 and 2.7 × 106 M−1. The luminescence spectra are also consistent with a limiting stoichiometry of 2.0 CuI/monomer (4 per dimer) as judged by point at which the maximum luminescence is obtained. At excess CuI, the luminescence intensity decreases significantly which is likely reporting on rearrangement of the chelate to a less solvent shielded environment concomitant with the formation of nonnative multinuclear copper clusters (38). This feature also characterizes CuI binding to some but not all unrelated copper regulatory proteins known to form multinuclear copper clusters (38, 47), but not in a mononuclear site of relatively low luminescence intensity (37). We see virtually the same luminescence titration curve with α5Δ BxmR (data not shown), revealing that not surprisingly, the carboxylate/imidazole-containing α5 site does not bind CuI, at least in a way that contributes to the luminescence intensity. Thus, unlike the case for the ZnII/CdII, these studies reveal that the α3N site of wild-type and C31S BxmRs binds CuI with a maximal stoichiometry of 2:1 (4 per dimer). We also note the CuI-saturated forms of wild-type and C31S BxmR contain a fully accessible α5 site since each is capable of binding CoII to this site (Supplementary Figure S4); this result suggests that these two classes of metal sites are structurally independent.
Figure 4.
Anaerobic CuI binding properties of 10.5 µM (monomer) WT BxmR (panels A-B) and 11.2 µM (monomer) (panels C) or 10.0 µM C31S BxmR (monomer) (panel D) as monitored by UV absorption at 265 nm (panels A, C) or CuI luminescence (panels B, D) (38). Conditions: 10 mM Mes, 0.1 M NaCl, pH 6.3.
BxmR forms a binuclear CuI2-S4 complex
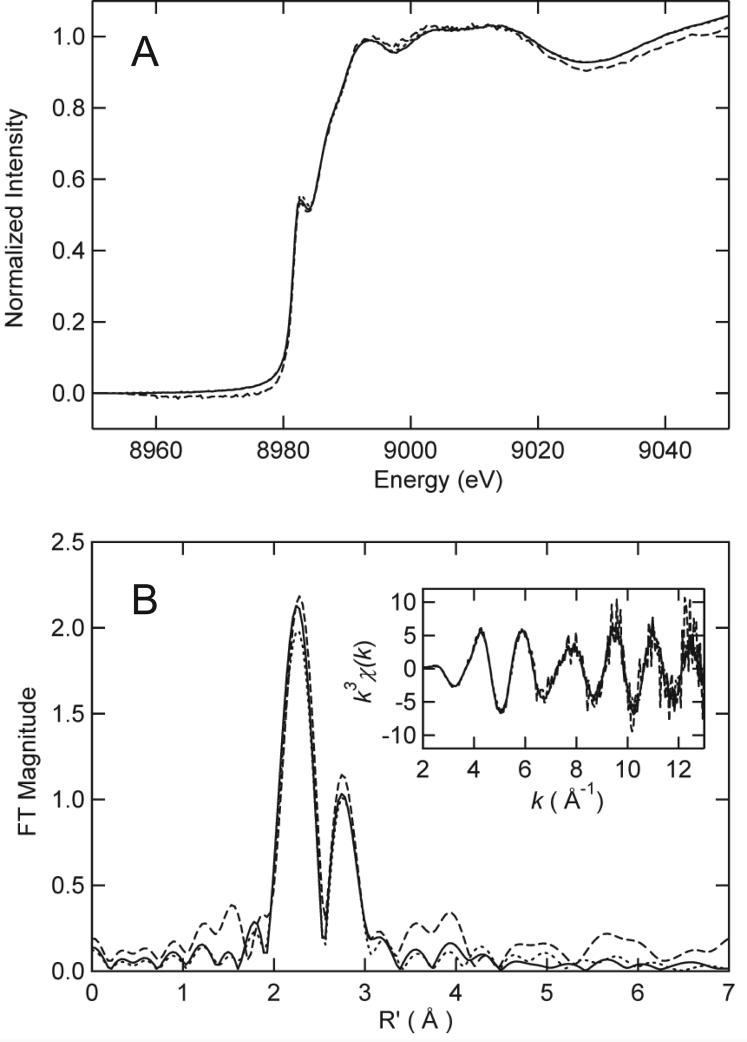
Copper K-edge X-ray absorption spectroscopy (XAS) was used to investigate the structure of the wild-type CuI complex formed at a 2:1 CuI:BxmR monomer molar ratio, and compared with that formed by C31S and α5Δ BxmRs, both at 1:1 metal-monomer ratios. The near edge spectra of all three samples are essentially superimposable and each is fully compatible with an average trigonally coordinated cuprous (CuI) ion (Figure 5A) (37). Experimental copper K-edge extended X-ray absorption fine structure (EXAFS) spectra are shown in the inset to Figure 5B with Fourier transforms of these data shown in the main body of the figure; structural parameters derived from the EXAFS curve fitting compiled in Supplementary Table S1. Virtually identical coordination environments are obtained for all three samples and each is well-described by three Cu-S interactions at 2.25-2.26 Å and an intense Cu-Cu scattering peak at ≈2.70 Å. These spectra are therefore consistent with a limiting CuI2-S4 complex, involving all four Cys of BxmR (Cys23, Cys31, Cys75 and Cys77) in wild-type and α5Δ BxmRs. Since the XAS near-edge and EXAFS spectra are virtually identical at saturating CuI for C31S BxmR this suggests that if Cys31 does indeed donate a thiolate ligand, it can be readily replaced with a molecule from solvent (H2O or Cl−; note that Cl− can not be distinguished from S−) (30), the Ser side chain itself, or another side chain, e.g., His27 (see Fig. 1). It is important to note that XAS near-edge and EXAFS spectra derived from coordination by a single His within a tetracopper CuI4•S5N complex could not be experimentally distinguished from CuI4•S6 complex (48); thus, recruitment or coordination by His27, if present, may be difficult to ascertain from these spectra of C31S BxmR.
Figure 5.
X-ray absorption spectroscopy of Cu-BxmRs. (A) Cu K-edge X-ray absorption near-edge spectra of WT BxmR (solid line), α5Δ BxmR (dashed line) and C31S BxmR (dotted line). (B) Cu—S phase-corrected EXAFS Fourier transforms of WT BxmR (solid line), α5Δ BxmR (dashed line) and C31S BxmR (dotted line). Inset, k3-weighted EXAFS spectra for each CuI-complex. Parameters that derive fitting these data to various coordination models are compiled in Supplementary Table S1.
AgI forms a mononuclear trigonal planar structure that is distinct from the CdII complex
In contrast to CuI, titration of apo-BxmR with AgI gives rise to significant absorption in the UV region that appears to be saturable with just one mol•equiv of the ion (spectra not shown). Perturbed angular correlation (PAC) spectroscopy was used to investigate the structure of the AgI complex in more detail and determine how this structure differs from that of CdII complex. 111Ag decays to 111mCd and in the PAC measurement the nuclear quadrupole interaction (NQI)1 between the cadmium nucleus and the surrounding charge distribution is recorded (49). It is assumed that the electronic relaxation after the nuclear decay is very fast on the PAC (nsec) timescale, but the structural relaxation from AgI coordination geometry to CdII coordination geometry may appear fast, intermediate or slow on this timescale. This can be tested by comparing 111Ag and 111mCd PAC spectra on the same protein. If they differ from one another, it is an indication that the structural relaxation is slow. Inspection of Fig. 6A-C reveals that there appears to be no trace of the 111mCd-BxmR PAC spectrum (Fig. 6C) in the 111Ag-BxmR PAC spectrum (Fig. 6A,B) ; this suggests that the lifetime of the AgI-coordination geometry is sufficiently long as to allow for observation of the AgI-coordination complex without complications of decay to the CdII complex.
Figure 6.
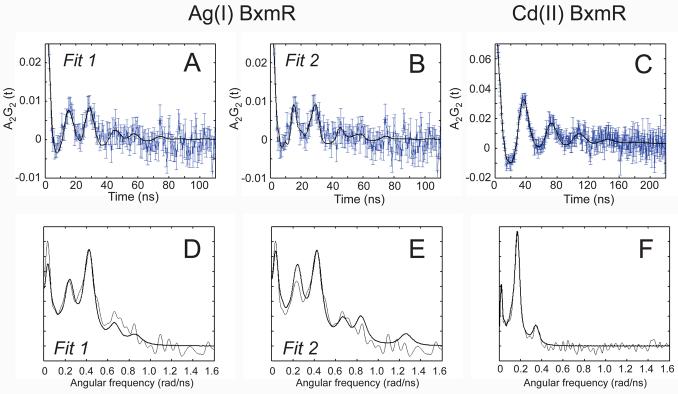
111Ag and 111mCd Perturbed Angular Correlation (PAC) spectroscopy of AgI-substituted and CdII-substituted wild-type (WT) BxmR, respectively. Top (panels A and B, 111Ag PAC; panel C, 111mCd PAC), the experimentally determined perturbation function (with error bars, in blue), and fit (bold faced black line). Bottom (panels D-F), Fourier transformations of the experimental perturbation function in panels A-C, respectively (thin line), and fit (bold faced line). The data from 111Ag PAC experiments in panels A and B are identical and merely show the results of two independent fits (1 and 2; see Table 2); these are multiplied by −1 to allow for easy comparison with 111mCd PAC data (panel C). Parameters that derive from the fitted curves are compiled in Table 2.
The Fourier transform of the AgI-BxmR perturbation function (Fig. 6D-E) reveals two main features, one at 0.23 rad/ns and one at ≈0.4 rad/ns. Since the ≈0.4 rad/ns peak has a higher amplitude than the peak at about 0.23 rad/ns, this strongly indicates that more than one NQI and thus more than one coordination environment is present in the AgI complex. The NQI with ω0 about 0.220 rad/ns (Table 2), can be extracted with reasonable certainty, as all three peaks (at about 0.220 rad/ns, 0.410 rad/ns and 0.630 rad/ns, respectively) are present beyond the noise in the Fourier transform, and they obey the rule ω1 + ω2 = ω3; this is valid as the intermediate nuclear level has a spin of 5/2 (50). The second NQI shown in Table 2 is less reliable and difficult to extract, and two possibilities (Fit 1 and Fit 2; Fig. 6D-E) give almost the same reduced χ2 when fit. These two NQIs both reproduce the low intensity peak at about 0.820 rad/ns observed at approximately twice the frequency of the most intense peak in the Fourier transform. This indicates that the corresponding NQI either has η close to 1 (Table 2 and Fig. 6D, Fit 1) or η close to 0 (Table 2 and Fig. 6E, Fit 2). It is a classic problem in PAC spectroscopy that these two cases are difficult to distinguish (51).3 The fact that two significantly different NQIs are observed reveals two different coordination environments are present in solution for AgI-BxmR, at least at AgI:protein ratio is about 1:10. Although the NQIs with ω0 at 0.220 rad/ns may represent a variety of coordination geometries, the NQI with ω0 about 0.420 rad/ns and low η is diagnostic for a trigonal planar coordination geometry composed of three coordinating cysteines.4
Table 2.
PAC fitted parameters for 111Ag and 111mCd bound to O. brevis BxmR.
| Protein | ω0 (rad/ns) |
η | Δω0/ω0 ×100 |
A ×100 |
χr2 |
|---|---|---|---|---|---|
| Ag-BxmR: Fit 1 | 0.217(3) | 0.33(4) | 2f | 2.1(3) | 1.11 |
| 0.245f | 1f | 2f | 1.6(4) | ||
| Ag-BxmR: Fit 2 | 0.221(3) | 0.32(3) | 2f | 3.0(2) | 1.19 |
| 0.420f | 0.05f | 2f | 1.8(2) | ||
| Cd-BxmR | 0.97(4) | 0.78(19) | 19(3) | 5.0(6) | 1.14 |
| 0.101(1) | 0.89(3) | 6(1) | 2.9(6) |
The numbers in parentheses represent the standard deviations of the fitted parameters. “f” indicates that the parameter was fixed in the fit.
Although visual inspection of the Cd-BxmR spectrum (Fig. 6C, F) seems to be indicative of only one NQI with a relatively high value of η, it is not possible to make a satisfactory fit the data with just one NQI. Indeed, addition of a second NQI reduced χ2 is reduced significantly (51) (by about 0.4). Thus the fit was carried out with two NQIs which, not surprisingly, turn out to be very similar (Table 2). This indicates that the two symmetry-related α3N sites in CdII-BxmR adopt similar coordination geometries at a CdII:protein ratio or ≈1:5. The relatively low values of ω0 might indicate the coordination geometries deviate markedly from perfect tetrahedral symmetry, assuming that only cysteine ligands are present (see Figure S2).
Regulation of operator-promoter (O/P) DNA binding of BxmR by different metal ions
The above metal binding experiments with wild-type and mutant BxmRs establish the stoichiometry and affinity by which individual metal ions bind to the α3N and α5 sites on BxmR, as well as insights into their coordination environments. Previous studies establish that BxmR binds to a typical ArsR-family operator containing a 12-2-12 inverted repeat sequence situated just upstream of the bxa1 gene encoding a CPx-ATPase (36). Fig. 7 shows representative anisotropy-based bxa1 O/P isotherms for wild-type (Fig. 7A), α3NΔ BxmR (Fig. 7B) and α5Δ BxmR (Fig. 7C) carried out with apo-BxmR or with BxmRs preincubated with 1:1 (ZnII, CdII or AgI) or 2:1 (CuI) (per BxmR monomer) of the indicated metal ion (25 ºC, pH 7.0, 0.2 M NaCl). The data were fit to a model assuming that two BxmR dimers bind to the operator with equal affinities (36), with the fitted parameters compiled in Table 3.
Figure 7.
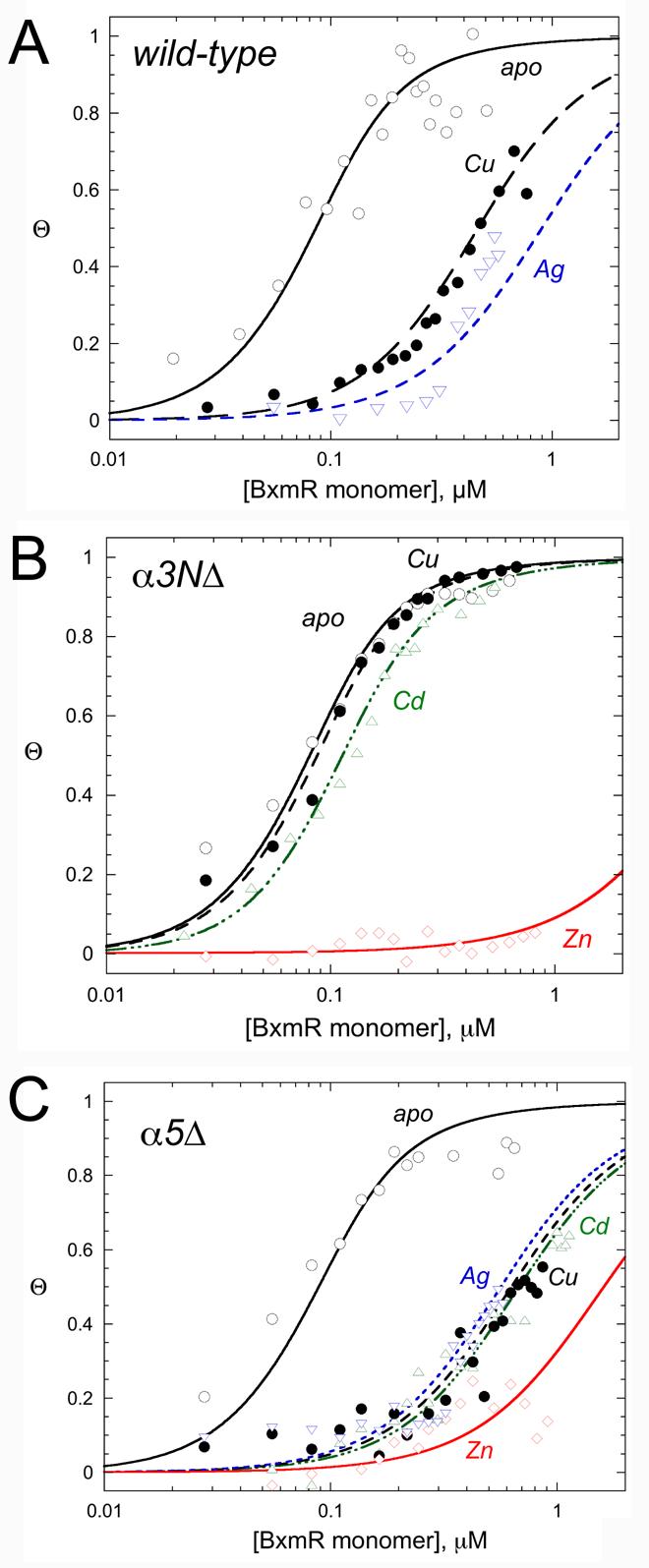
Representative normalized fluorescence anisotropy-based bxa1 operator/promoter DNA binding isotherms for various WT (A), α3NΔ (B) and α5Δ (C) BxmRs in the absence (apo-) and presence of the indicated metal ions. The continuous line through each binding isotherm represents a nonlinear least squares fit to a two-dimer DNA binding model (see Experimental Procedures), with the fitted parameters compiled in Table 3.
Table 3.
bxa1 O/P binding parameters for apo- and various metal loaded states of wild-type, α3NΔ and α5Δ BxmRsa
| BxmR protein | metal | rD | r(P2)2·D | K1 (M−1) | ΔGc (kcal/mol)b |
|---|---|---|---|---|---|
| wild-type | apo | 0.108 | 0.135 | 1.0 (±0.2) × 108 | – |
| CuI1.8 | 0.106 | 0.135c | 7.1 (±0.1) × 106 | 1.6 | |
| AgI | 0.108 | 0.135c | 3.7 (±0.4) × 106 | 1.9 | |
| α3NΔ | apo | 0.108 | 0.138 | 1.2 (±0.1) × 108 | – |
| CuI | 0.109 | 0.138 | 1.0 (±0.2) × 108 | 0.1 | |
| CdII | 0.113 | 0.135 | 7.2 (±0.1) × 107 | 0.3 | |
| ZnII | 0.109 | 0.138c | ≤5.0 (±0.3) × 105 | ≥3.2d | |
| α5Δ | apo | 0.109 | 0.137 | 1.0 (±0.2) × 108 | – |
| CuI | 0.106 | 0.137c | 5.0 (±0.1) × 106 | 1.8 | |
| AgI | 0.105 | 0.137c | 5.6 (±0.1) × 106 | 1.7 | |
| CdII | 0.111 | 0.137c | 5.0 (±0.1) × 106 | 1.8 | |
| ZnII | 0.110 | 0.137c | 1.8 (±0.4) × 106 | 2.4 |
Parameters determined from nonlinear least squares fits the data shown in Fig. 7 using the model described under Experimental Procedures.
Estimated uncertainty in ΔGc is ±0.1 kcal/mol.
This value of r(P2)2·D was fixed to the value obtained for the appropriate apoprotein in each case during the parameter optimization. The value of rD was fixed to the experimentally measured value during parameter optimization.
Reflects a lower limit given the upper limit on K1 (see Fig. 7B for binding curve).
The data taken collectively reveal that bxa1 O/P DNA binding activity of BxmR is allosterically negatively regulated by all four metals tested. Monovalent CuI and AgI as well as divalent CdII are equally effective in inhibiting DNA binding, with an allosteric coupling free energy, ΔGc, of +1.7 (±0.1) kcal mol−1 under these conditions (Table 3). All three of these metals function through the thiophilic α3N metal binding site, since α3NΔ BxmR shows no regulation by CdII or CuI (Fig. 7B). In striking contrast to CuI and CdII, ZnII is unique in that this metal is a potent effector of bxa1 O/P binding by α3NΔ BxmR, with a lower limit for ΔGc, of +3.4 (±0.1) kcal mol−1; this result shows that ZnII is capable of functioning through the α5 metal binding sites. Binding to the α5 sites is not obligatory however, since ZnII also negatively regulates DNA binding in α5Δ BxmR, i.e., through the α3N binding sites. Thus, BxmR retains a functional redundancy in its response to ZnII, unlike all other metals that induce bxa1 expression in O. brevis (32).
DISCUSSION
O. brevis BxmR is the fifth primary zinc-sensing member of the ArsR/SmtB family of metalloregulatory repressors (33) to be purified and characterized, four of which derive from different cyanobacterial species. The others are Synechococcus SmtB (17), Synechocystis ZiaR (52), Anabaena PCC7120 AztR (8) and S. aureus CzrA (20, 53). BxmR is most closely related on the basis of global amino acid sequence similarity to ZiaR, followed by AztR and SmtB (see Figs. 1, 8A), and these four proteins are part of a small cluster of sequences within the Group 2 clade of ArsR-family repressors (7) (our data not shown; see Fig. 8A). These sensors represent four of the ≈15 ArsR/SmtB sensors for which significant biochemical and cell biological data are now available, which collectively respond to an impressive range of metal ions including ZnII, ZnII/CoII, NiII/CoII, CdII/PbII, AsIII/SbIII, BiIII and CuI/AgI (6, 14) (see Fig. 8B). In this report, we establish that BxmR is unique in this family of proteins in that despite the fact that ZnII is the most potent inducer of bxa1 expression (33), BxmR mediates a clear response to CuI/AgI both intracellularly and in vitro (36) in striking contrast to ZiaR (52).
Figure 8.
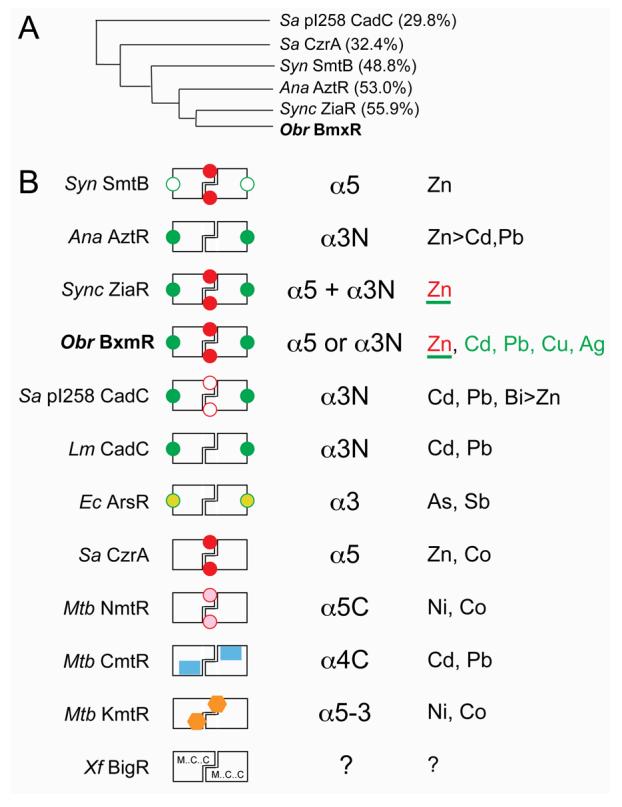
Schematic comparison of ArsR/SmtB family metal sensors with well-characterized metal binding properties. (A) Dendogram that illustrates the degree to which the four cyanobacterial ArsR/SmtB zinc sensors are related to one another and to previously characterized α5 (Sa CzrA) and α3N (Sa pI258 CadC) sensors on the basis of a global sequence similarity (% identity to Obr BxmR also indicated). (B) Schematic rendering of ArsR/SmtB sensors that highlights known regulatory metal sites (filled symbols) with their associated trivial designations (third column) and metal ions that are sensed in vivo (fourth column) by these sites. Other known metal sites that play no regulatory role are represented by open symbols (in Syn SmtB and Sa pI258 CadC). Sensors are: Synechococcus (Syn) SmtB (31, 57), Anabaena (Ana) AztR (8), Synechocystis (Sync) ZiaR (6, 52), O. brevis (Obr) BxmR (this work), S. aureus (Sa) pI258 CadC (30, 46), Listeria monocytogenes (Lm) CadC (30), E. coli R773 (Ec) ArsR (59), S. aureus (Sa) CzrA (3), M. tuberculosis (Mtb) NmtR (20, 60), M. tuberculosis (Mtb) CmtR (22, 24, 25), M. tuberculosis (Mtb) KmtR (7), Xylella fastidiosa (Xf) BigR (27, 29). For Obr BxmR, the metals sensed by the α3N site are shaded green; ZnII is sensed by both α5 and α3N sites (this work); for ZiaR, both sites are apparently required to sense ZnII in vivo (52). Although the inducer identity of Xf BigR is not yet known (?), BigR contains conserved Met, Cys and Cys residues in the projected α1, α2 and α5 helices (27). See text for additional details.
Extensive biochemical, crystallographic and NMR structural studies of CzrA, a ZnII/CoII sensor from S. aureus, the CdII/PbII sensor CadC from S. aureus pI258 and L. monocytogenes, Synechococcus SmtB and NmtR, the NiII/CoII sensor from M. tuberculosis, establishes the evolution of two distinct metal sensing sites (α3N and α5) on this homodimeric winged helical scaffold (Fig. 8B) (1, 3, 20, 21, 26, 30). The α3N site is anchored by a pair of Cys likely positioned at the N-terminus of the α3 helix (Cys75 and Cys77 in BxmR), but shows considerable variability in the number and type of donor atoms that are known or predicted to make coordination bonds to the metal. Previous studies of S. aureus CadC established that a Gly substitution of the Cys corresponding to Cys77 in BxmR abrogates allosteric coupling of CdII/PbII/ZnII binding to DNA binding by CadC and rendered CadC unable to sense metals in vivo (45); the analogous Ser substitution in AztR abolishes ZnII sensing in vivo and PbII binding in vitro (8). Interestingly, the same Gly substitution occurs naturally in SmtB (see Fig. 1); this substitution makes the prediction that the α3N site in SmtB would be capable of binding metals, but would not be regulatory and this is exactly in accord with experiment (31). Interestingly, S. aureus CadC contains both intact α3N and α5 metal sites, and the crystal structure of “apo-CadC” revealed that both α5 sites were filled with ZnII (1), a finding consistent with its very high equilibrium affinity (L. Busenlehner and D. Giedroc, unpublished results). However, this site is not metalloregulatory for cad O/P binding, since an α3N inactivating mutation analogous to the one characterized here in α5Δ BxmR (C60G in CadC; C77S in BxmR) abolishes regulation of DNA binding by all metal ions (30). This property is in strong contrast to BxmR which retains potent ZnII regulation through its α5 metal sites to a degree that is comparable to that of bona fide α5 zinc sensor S. aureus CzrA (23, 41).
It is striking that the four cyanobacterial zinc sensors have evolved distinct metal binding properties in a way that seems to track largely with differences in the N-terminal region. CadC contains an additional N-terminal α-helix (α0)1 not found α5 sensors SmtB (3), CzrA (54) or NmtR (A. Arunkumar, H. Reyes, and D. Giedroc, unpublished observations), with the most N-terminal residue visible in electron density maps Tyr12 (1). Tyr12 is just C-terminal to Cys7 and Cys11, the former of which donates a thiolate ligand to the CdII/PbII ion (30) and may well be required to drive a conformational change in the dimer that lowers the DNA-binding affinity of the repressor (55). ZiaR and AztR contain longer N-terminal domains (see Fig. 1). Each contains a single Cys that aligns with the key allosteric residue Cys7 of CadC, as well as one conserved His (His27 in AztR). The α3N chelates of ZiaR and AztR are structurally indistinguishable and are consistent with an S3(N/O) tetrahedral coordination environment as determined by the CoII coordination complex, easily distinguished from the highly distorted S4 site of CadC (8). BxmR contains the longest N-terminal extension of any cyanobacterial ArsR-family ZnII sensor to date (see Fig. 1). BxmR conserves the key Cys corresponding to Cys7 in CadC (Cys23), but also contains three additional candidate metal ligands: a His analogous to His18 in SmtB (this His is proposed to donate a ligand to the α3N-bound ZnII in SmtB (21, 43)), a unique Cys (Cys31) not found in the other sensors, and His15, analogous to Cys12 in ZiaR (see Fig. 1). The CoII spectrum of CoII-substituted α5Δ BxmR is very similar to CoII-AztR, and is thus consistent with S3(N/O) α3N site (8); however, the CdII complex contains 3-4 thiolate ligands, with the unique Cys31 apparently making a coordination bond (Supplementary Figures S2-S3).
The distinct N-terminal region of BxmR stably accommodates an α3N CuI complex in a solvent-shielded (38) binuclear Cu2S4 cluster that we propose involves Cys23, Cys31, Cys75′, Cys77′ as wild-type metal ligands. This proposed binuclear copper cluster structure appears analogous to that proposed for CuI-specific sensor Enterococcus CopY (56). Each of the CuI ions in BxmR is trigonally coordinated with three Cu-S− distances at 2.26 Å and a single Cu-Cu distance of 2.70 Å; see Fig. 5); essentially identical spectra are obtained with the WT and α5Δ BxmR consistent with the conclusion that the C-terminal α5 site plays no role in CuI binding or CuI-dependent negative allosteric regulation of DNA binding (Fig. 7). We note however, that CuI binds to BxmR with an average step-wise binding affinity of ≈1 × 107 M−1 (Fig. 4A), which is quite weak relative to other known mononuclear bacterial CuI sensors E. coli CueR (19) and M. tuberculosis CsoR (37) as well as the multinuclear CuI complex in Drosophila MTF-1 (38). This finding is consistent with the idea that the α3N site in BxmR is not selective for CuI and may have resulted from the use of copper salts as algaecides for O. brevis used in these studies (33). These physical data show conclusively that residues derived from the N-terminal domain of BxmR allow direct binding of monovalent ions CuI/AgI in a way that induces negative regulation of DNA binding to a degree that is identical to divalent ions ZnII/CdII. These findings provide additional evidence that the α3N site is structurally plastic, and particularly well-suited to adopt a wide range of coordination chemistries (geometries and stoichiometries) to accommodate a wider range of metal ions and thereby mediate their homeostasis under conditions of metal ion excess (30).
A side-by-side comparison of the physical properties of the four closely related zinc-sensing ArsR/SmtB repressors (Syn SmtB, Ana AztR, Obr BxmR and Sync ZiaR) reveals the remarkable finding that each likely utilizes distinct structural mechanism to mediate resistance to toxic concentrations of ZnII in the cell (see Fig. 8). A similar conclusion was reached on the basis of a comparison of three AsIII-sensing ArsRs, each of which harbor distinct S3 coordination sites proposed to result from convergent evolution in response to recent environmental pressures (5). We note that Anabaena contains one other ArsR/SmtB regulator, most closely related to Syn SmtB termed AzuR (alr0831) not listed in Fig. 8B; AzuR has been proposed to be a ZnII uptake repressor (8) but this has not yet been tested experimentally.5 In any case, Syn SmtB (and likely Ana AzuR) utilizes a single pair of subunit bridging α5 metal sites (31, 57) much like Sa CzrA (23). In AztR, the α5 metal sites have been lost and as a result, ZnII regulation falls to the CadC-like S3(N/O) α3N sites (8). AztR also senses CdII/PbII through these metal sites, but the metal effluxer whose expression is regulated by AztR, AztA, is largely functionally selective for ZnII (58). In contrast, Obr BxmR can exploit either α5 or α3N metal sites to effect zinc regulation, with the ZnII-bound α5 sites far more selective and far more effective in allosterically inhibiting operator/promoter DNA binding. Finally, in vivo experiments suggest that Sync ZiaR requires both α5 or α3N metal sites to effect metalloregulation by ZnII since inactivation of one or the other abolished ZnII sensing in the cell (52); the structural basis for this is not yet known but would appear to be in contrast to BxmR. This feature might enhance the metal selectivity of ZiaR for ZnII relative to AztR and BxmR since CuI and AgI do not induce the zia operon in Synechocystis cultures (52). It will be interesting to determine the extent to which the metallothionein BmtA and the metal transporter Bxa1 binds and transports, respectively, AgI/CuI vs. ZnII/CdII.
One common feature of all ArsR/SmtB sensors that have investigated in detail to date is that filling of one of two symmetry-related metal sites is necessary and sufficient to mediate regulation of DNA binding (41). For example, inactivation of one of the two pairs of sensing sites in BxmR to create α5Δ and α3NΔ BxmRs results in retention of a single high affinity metal binding site with the second symmetry-related site on the homodimer characterized by a lower affinity for the metal (Table 1); a similar characteristic was found for all α5 first coordination shell mutants of Sa CzrA (23). Nonetheless, all drive approximately wild-type levels of regulation, at least in vitro. Current efforts are directed toward understanding in molecular terms how metal binding to the α5 vs. the α3N sites drives negative allosteric regulation of DNA binding in BxmR and other ArsR family sensors, and what factors dictate the quantitatively more potent regulation that occurs upon metal binding by the C-terminal α5 sites (54).
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr. Larry Dangott in the Protein Chemistry Laboratory at Texas A&M University for performing amino acid analysis on BxmR samples.
This work was supported by grants from the National Institutes of Health (GM042569) and the Robert A. Welch Foundation (A-1295) to D. P. G.
Footnotes
Abbreviations used are as follows: BxmR, O. brevis CPx-ATPase metal-regulated repressor; Bxa1, O. brevis CPx-ATPase-1; BmtA, O. brevis metallothionein-A; ArsR, arsenic repressor; mag-fura-2, 2-[6-[bis(carboxymethyl)amino]-5-(carboxymethoxy)-2-benzofuranyl]- 5-oxazolecarboxylic acid; quin-2, N-(2-((8-(bis(carboxymethyl)amino)-6-methoxy-2-quinolinyl)methoxy)-4-methylphenyl)-N-(carboxymethyl)-glycine; NQI, nuclear quadrupole interaction.
S. aureus pI258 CadC (1)contains an additional N-terminal α-helix relative to zinc sensors Synechococcus SmtB and S. aureus CzrA (2, 3). Thus the helical designations α1-α5 in SmtB correspond to helices α2-α6 in CadC and ArsRs recently characterized (4, 5). The designation of metal sites used here, e.g. α3N, conforms to the SmtB/CzrA secondary structure convention used in nearly all of the previous work (6, 7).
Other fits may give equally good reduced χ2 values, but will not reproduce the low intensity peak at about 0.82 rad/ns in Fig. 6D, E. A number of parameters were fixed in the fit in order to force the fit to include the considerations described in the text. Floating all parameters did not significantly change the reduced χ2.
We note that as a control, we also carried out a 111Ag PAC experiment on precipitated AgCl. This spectrum is clearly different (data not shown) from that observed in the Ag-BxmR; thus, it is reasonable to assume that the PAC signal deriving the AgI-BxmR complex does indeed originate from AgI bound to BxmR.
Like SmtB, AzuR contains a naturally occurring non-liganding Ser in the α3 CVS sequence (analogous to C74S AztR (8) and C77S BxmR characterized here); this is predicted to inactivate ZnII sensing at this site. Recent studies show that AzuR binds a single monomer mol•equiv of ZnII/CoII with high affinity to form a tetrahedral (N/O)4 α5 site complex and is therefore structurally indistinguishable from Sa CzrA (Z. Ma, R. Scott and D. Giedroc, unpublished observations) (3).
SUPPORTING INFORMATION AVAILABLE
This information includes Supplementary Methods, the curve-fitting results from the CuI EXAFS experiments (Table S1), ZnII-binding data for the α3NΔ and α5Δ BxmRs (Figure S1); spectral data for the CoII- (Figure S2) and CdII- (Figure S3) substituted wild-type and mutant BxmRs; and the spectrum obtained after 1.1 mol•equiv CoII is added CuI2-C31S BxmR (Figure S4). This material is available free of charge via the Internet at http://pbs.acs.org.
REFERENCES
- 1.Ye J, Kandegedara A, Martin P, Rosen BP. Crystal structure of the Staphylococcus aureus pI258 CadC Cd(II)/Pb(II)/Zn(II)-responsive repressor. J Bacteriol. 2005;187:4214–4221. doi: 10.1128/JB.187.12.4214-4221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook WJ, Kar SR, Taylor KB, Hall LM. Crystal structure of the cyanobacterial metallothionein repressor SmtB: a model for metalloregulatory proteins. J Mol Biol. 1998;275:337–346. doi: 10.1006/jmbi.1997.1443. [DOI] [PubMed] [Google Scholar]
- 3.Eicken C, Pennella MA, Chen X, Koshlap KM, VanZile ML, Sacchettini JC, Giedroc DP. A metal-ligand-mediated intersubunit allosteric switch in related SmtB/ArsR zinc sensor proteins. J Mol Biol. 2003;333:683–695. doi: 10.1016/j.jmb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, Fu HL, Ye J, Bencze KZ, Stemmler TL, Rawlings DE, Rosen BP. Convergent evolution of a new arsenic binding site in the ArsR/SmtB family of metalloregulators. J Biol Chem. 2007;282:34346–34355. doi: 10.1074/jbc.M706565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordoñez E, Thiyagarajan S, Cook JD, Stemmler TL, Gil JA, Mateos LM, Rosen BP. Evolution of metal(loid) binding sites in transcriptional regulators. J Biol Chem. 2008:283. doi: 10.1074/jbc.M803209200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busenlehner LS, Pennella MA, Giedroc DP. The SmtB/ArsR family of metalloregulatory transcriptional repressors: Structural insights into prokaryotic metal resistance. FEMS Microbiol Rev. 2003;27:131–143. doi: 10.1016/S0168-6445(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DR, Chapman KE, Waldron KJ, Tottey S, Kendall S, Cavallaro G, Andreini C, Hinds J, Stoker NG, Robinson NJ, Cavet JS. Mycobacterial cells have dual nickel-cobalt sensors: sequence relationships and metal sites of metal-responsive repressors are not congruent. J Biol Chem. 2007;282:32298–32310. doi: 10.1074/jbc.M703451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Golden JW, Giedroc DP. A zinc(II)/lead(II)/cadmium(II)-inducible operon from the cyanobacterium Anabaena is regulated by AztR, an α3N ArsR/SmtB metalloregulator. Biochemistry. 2005;44:8673–8683. doi: 10.1021/bi050450+. [DOI] [PubMed] [Google Scholar]
- 9.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 10.O'Halloran TV. Transition metals in control of gene expression. Science. 1993;261:715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- 11.Finney LA, O'Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 12.Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 13.Barkay T, Miller SM, Summers AO. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol Rev. 2003;27:355–384. doi: 10.1016/S0168-6445(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 14.Giedroc DP, Arunkumar AI. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 2007:3107–3120. doi: 10.1039/b706769k. [DOI] [PubMed] [Google Scholar]
- 15.Hobman JL, Wilkie J, Brown NL. A design for life: prokaryotic metal-binding MerR family regulators. Biometals. 2005;18:429–436. doi: 10.1007/s10534-005-3717-7. [DOI] [PubMed] [Google Scholar]
- 16.San Francisco MJ, Hope CL, Owolabi JB, Tisa LS, Rosen BP. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic acids research. 1990;18:619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morby AP, Turner JS, Huckle JW, Robinson NJ. SmtB is a metal-dependent repressor of the cyanobacterial metallothionein gene smtA: identification of a Zn inhibited DNA-protein complex. Nucleic acids research. 1993;21:921–925. doi: 10.1093/nar/21.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwig JS, Leitch S, Herbst RW, Maroney MJ, Chivers PT. Ni(II) and Co(II) sensing by Escherichia coli RcnR. J Am Chem Soc. 2008;130:7592–7606. doi: 10.1021/ja710067d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Changela A, Chen K, Xue Y, Holschen J, Outten CE, O'Halloran TV, Mondragon A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301:1383–1387. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- 20.Pennella MA, Shokes JE, Cosper NJ, Scott RA, Giedroc DP. Structural elements of metal selectivity in metal sensor proteins. Proc Natl Acad Sci U S A. 2003;100:3713–3718. doi: 10.1073/pnas.0636943100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanZile ML, Chen X, Giedroc DP. Structural characterization of distinct α3N and α5 metal sites in the cyanobacterial zinc sensor SmtB. Biochemistry. 2002;41:9765–9775. doi: 10.1021/bi0201771. [DOI] [PubMed] [Google Scholar]
- 22.Cavet JS, Graham AI, Meng W, Robinson NJ. A cadmium-lead-sensing ArsR-SmtB repressor with novel sensory sites. Complementary metal discrimination by NmtR and CmtR in a common cytosol. J Biol Chem. 2003;278:44560–44566. doi: 10.1074/jbc.M307877200. [DOI] [PubMed] [Google Scholar]
- 23.Pennella MA, Arunkumar AI, Giedroc DP. Individual metal ligands play distinct functional roles in the zinc sensor Staphylococcus aureus CzrA. J Mol Biol. 2006;356:1124–1136. doi: 10.1016/j.jmb.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Hemmingsen L, Giedroc DP. Structural and functional characterization of Mycobacterium tuberculosis CmtR, a PbII/CdII-sensing SmtB/ArsR metalloregulatory repressor. Biochemistry. 2005;44:8976–8988. doi: 10.1021/bi050094v. [DOI] [PubMed] [Google Scholar]
- 25.Banci L, Bertini I, Cantini F, Ciofi-Baffoni S, Cavet JS, Dennison C, Graham AI, Harvie DR, Robinson NJ. NMR structural analysis of cadmium sensing by winged helix repressor CmtR. J Biol Chem. 2007;282:30181–30188. doi: 10.1074/jbc.M701119200. [DOI] [PubMed] [Google Scholar]
- 26.Pennella MA, Giedroc DP. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals. 2005;18:413–428. doi: 10.1007/s10534-005-3716-8. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa RL, Benedetti CE. BigR, a transcriptional repressor from plant-associated bacteria, regulates an operon implicated in biofilm growth. J Bacteriol. 2007;189:6185–6194. doi: 10.1128/JB.00331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rother D, Orawski G, Bardischewsky F, Friedrich CG. SoxRS-mediated regulation of chemotrophic sulfur oxidation in Paracoccus pantotrophus. Microbiology. 2005;151:1707–1716. doi: 10.1099/mic.0.27724-0. [DOI] [PubMed] [Google Scholar]
- 29.Barbosa RL, Rinaldi FC, Guimaraes BG, Benedetti CE. Crystallization and preliminary X-ray analysis of BigR, a transcription repressor from Xylella fastidiosa involved in biofilm formation. Acta crystallographica. 2007;63:596–598. doi: 10.1107/S1744309107028722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busenlehner LS, Weng TC, Penner-Hahn JE, Giedroc DP. Elucidation of primary (α3N) and vestigial (α5) heavy metal-binding sites in Staphylococcus aureus pI258 CadC: evolutionary implications for metal ion selectivity of ArsR/SmtB metal sensor proteins. J Mol Biol. 2002;319:685–701. doi: 10.1016/S0022-2836(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 31.VanZile ML, Chen X, Giedroc DP. Allosteric negative regulation of smt O/P binding of the zinc sensor, SmtB, by metal ions: a coupled equilibrium analysis. Biochemistry. 2002;41:9776–9786. doi: 10.1021/bi020178t. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Nakashima S, Hirose K, Uemura Y, Shibasaka M, Katsuhara M, Kasamo K. A metallothionein and CPx-ATPase handle heavy-metal tolerance in the filamentous cyanobacterium Oscillatoria brevis. FEBS Lett. 2003;542:159–163. doi: 10.1016/s0014-5793(03)00370-3. [DOI] [PubMed] [Google Scholar]
- 33.Tong L, Nakashima S, Shibasaka M, Katsuhara M, Kasamo K. A novel histidine-rich CPx-ATPase from the filamentous cyanobacterium Oscillatoria brevis related to multiple-heavy-metal cotolerance. J Bacteriol. 2002;184:5027–5035. doi: 10.1128/JB.184.18.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrader KK, Nanayakkara NP, Tucker CS, Rimando AM, Ganzera M, Schaneberg BT. Novel derivatives of 9,10-anthraquinone are selective algicides against the musty-odor cyanobacterium Oscillatoria perornata. Appl Environ Microbiol. 2003;69:5319–5327. doi: 10.1128/AEM.69.9.5319-5327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirose K, Ezaki B, Liu T, Nakashima S. Diamide stress induces a metallothionein BmtA through a repressor BxmR and is modulated by Zn-inducible BmtA in the cyanobacterium Oscillatoria brevis. Tox Lett. 2006;163:250–256. doi: 10.1016/j.toxlet.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Liu T, Nakashima S, Hirose K, Shibasaka M, Katsuhara M, Ezaki B, Giedroc DP, Kasamo K. A novel cyanobacterial SmtB/ArsR family repressor regulates the expression of a CPx-ATPase and a metallothionein in response to both Cu(I)/Ag(I) and Zn(II)/Cd(II) J Biol Chem. 2004;279:17810–17818. doi: 10.1074/jbc.M310560200. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Hua H, Balamurugan K, Kong X, Zhang L, George GN, Georgiev O, Schaffner W, Giedroc DP. Copper sensing function of Drosophila metal-responsive transcription factor-1 is mediated by a tetranuclear Cu(I) cluster. Nucleic acids research. 2008;36:3128–3138. doi: 10.1093/nar/gkn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walkup GK, Imperiali B. Fluorescent chemosensors fr divalent zinc based on zinc finger domains. Enhanced oxidative stability, metal binding affinity, and structural and functional characterization. J Am Chem Soc. 1997;199:3443–3450. [Google Scholar]
- 40.Jefferson JR, Hunt JB, Ginsburg A. Characterization of indo-1 and quin-2 as spectroscopic probes for Zn2(+)-protein interactions. Anal Biochem. 1990;187:328–336. doi: 10.1016/0003-2697(90)90465-l. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Arunkumar AI, Chen X, Giedroc DP. Structural insights into homo- and heterotropic allosteric coupling in the zinc sensor S. aureus CzrA from covalently fused dimers. J Am Chem Soc. 2006;128:1937–1947. doi: 10.1021/ja0546828. [DOI] [PubMed] [Google Scholar]
- 42.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 43.VanZile ML, Cosper NJ, Scott RA, Giedroc DP. The zinc metalloregulatory protein Synechococcus PCC7942 SmtB binds a single zinc ion per monomer with high affinity in a tetrahedral coordination geometry. Biochemistry. 2000;39:11818–11829. doi: 10.1021/bi001140o. [DOI] [PubMed] [Google Scholar]
- 44.Busenlehner LS, Cosper NJ, Scott RA, Rosen BP, Wong MD, Giedroc DP. Spectroscopic properties of the metalloregulatory Cd(II) and Pb(II) sites of S. aureus pI258 CadC. Biochemistry. 2001;40:4426–4436. doi: 10.1021/bi010006g. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Wong MD, Rosen BP. Role of cysteinyl residues in sensing Pb(II), Cd(II), and Zn(II) by the plasmid pI258 CadC repressor. J Biol Chem. 2001;276:14955–14960. doi: 10.1074/jbc.M010595200. [DOI] [PubMed] [Google Scholar]
- 46.Busenlehner LS, Apuy JL, Giedroc DP. Characterization of a metalloregulatory bismuth(III) site in Staphylococcus aureus pI258 CadC repressor. J Biol Inorg Chem. 2002;7:551–559. doi: 10.1007/s00775-001-0336-9. [DOI] [PubMed] [Google Scholar]
- 47.Cobine P, Wickramasinghe WA, Harrison MD, Weber T, Solioz M, Dameron CT. The Enterococcus hirae copper chaperone CopZ delivers copper(I) to the CopY repressor. FEBS Lett. 1999;445:27–30. doi: 10.1016/s0014-5793(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 48.Brown KR, Keller GL, Pickering IJ, Harris HH, George GN, Winge DR. Structures of the cuprous-thiolate clusters of the Mac1 and Ace1 transcriptional activators. Biochemistry. 2002;41:6469–6476. doi: 10.1021/bi0160664. [DOI] [PubMed] [Google Scholar]
- 49.Bauer R, Danielsen E, Hemmingsen L, Bjerrum MJ, Hansson O, Singh K. Interplay between oxidation state and coordination geometry of metal ions in azurin. J Am Chem Soc. 1997;119:157–162. [Google Scholar]
- 50.Hemmingsen L, Sas KN, Danielsen E. Biological applications of perturbed angular correlations of gamma-ray spectroscopy. Chem Rev. 2004;104:4027–4062. doi: 10.1021/cr030030v. [DOI] [PubMed] [Google Scholar]
- 51.Hemmingsen L, Hansen B. Bayes theorem applied to the selection of model in PAC spectroscopy. Z Natureforsch A. 1995;51a:442–446. [Google Scholar]
- 52.Thelwell C, Robinson NJ, Turner-Cavet JS. An SmtB-like repressor from Synechocystis PCC 6803 regulates a zinc exporter. Proc Natl Acad Sci U S A. 1998;95:10728–10733. doi: 10.1073/pnas.95.18.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroda M, Hayashi H, Ohta T. Chromosome-determined zinc-responsible operon czr in Staphylococcus aureus strain 912. Microbiol Immunol. 1999;43:115–125. doi: 10.1111/j.1348-0421.1999.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 54.Arunkumar AI, Pennella MA, Kong X, Giedroc DP. Resonance assignments of the metal sensor protein CzrA in the apo-. Zn2- and DNA-bound (42 kD) states. Biomol NMR Assign. 2007;1:99–101. doi: 10.1007/s12104-007-9027-y. [DOI] [PubMed] [Google Scholar]
- 55.Busenlehner LS, Giedroc DP. Kinetics of metal binding by the toxic metal-sensing transcriptional repressor Staphylococcus aureus pI258 CadC. Journal of inorganic biochemistry. 2006 doi: 10.1016/j.jinorgbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Cobine PA, George GN, Jones CE, Wickramasinghe WA, Solioz M, Dameron CT. Copper transfer from the Cu(I) chaperone, CopZ, to the repressor, Zn(II)CopY: metal coordination environments and protein interactions. Biochemistry. 2002;41:5822–5829. doi: 10.1021/bi025515c. [DOI] [PubMed] [Google Scholar]
- 57.Turner JS, Glands PD, Samson AC, Robinson NJ. Zn2+-sensing by the cyanobacterial metallothionein repressor SmtB: different motifs mediate metal-induced protein-DNA dissociation. Nucleic acids research. 1996;24:3714–3721. doi: 10.1093/nar/24.19.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu T, Reyes-Caballero H, Li C, Scott RA, Giedroc DP. Multiple metal binding domains enhance the Zn(II) selectivity of the divalent metal ion transporter AztA. Biochemistry. 2007;46:11057–11068. doi: 10.1021/bi7006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi W, Dong J, Scott RA, Ksenzenko MY, Rosen BP. The role of arsenic-thiol interactions in metalloregulation of the ars operon. J Biol Chem. 1996;271:9291–9297. doi: 10.1074/jbc.271.16.9291. [DOI] [PubMed] [Google Scholar]
- 60.Cavet JS, Meng W, Pennella MA, Appelhoff RJ, Giedroc DP, Robinson NJ. A nickel-cobalt-sensing ArsR-SmtB family repressor. Contributions of cytosol and effector binding sites to metal selectivity. J Biol Chem. 2002;277:38441–38448. doi: 10.1074/jbc.M207677200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.