Figure 8.
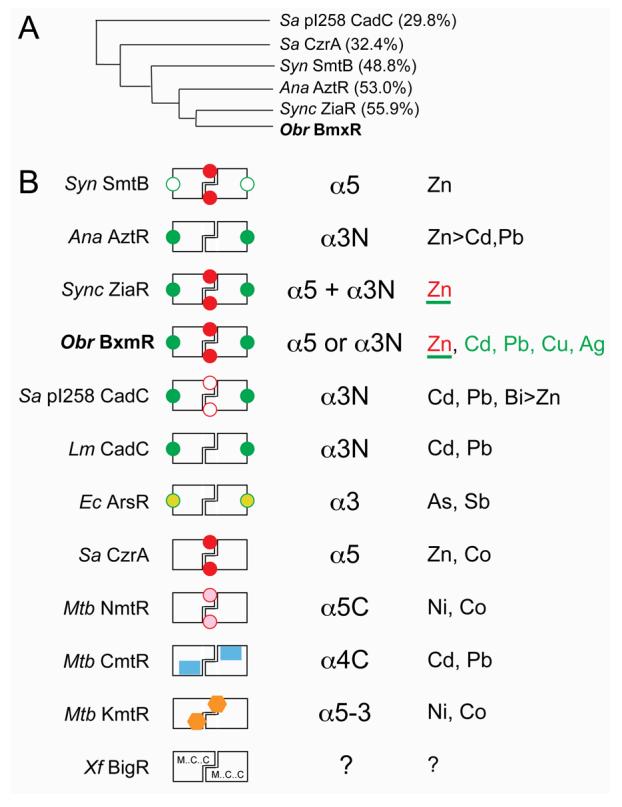
Schematic comparison of ArsR/SmtB family metal sensors with well-characterized metal binding properties. (A) Dendogram that illustrates the degree to which the four cyanobacterial ArsR/SmtB zinc sensors are related to one another and to previously characterized α5 (Sa CzrA) and α3N (Sa pI258 CadC) sensors on the basis of a global sequence similarity (% identity to Obr BxmR also indicated). (B) Schematic rendering of ArsR/SmtB sensors that highlights known regulatory metal sites (filled symbols) with their associated trivial designations (third column) and metal ions that are sensed in vivo (fourth column) by these sites. Other known metal sites that play no regulatory role are represented by open symbols (in Syn SmtB and Sa pI258 CadC). Sensors are: Synechococcus (Syn) SmtB (31, 57), Anabaena (Ana) AztR (8), Synechocystis (Sync) ZiaR (6, 52), O. brevis (Obr) BxmR (this work), S. aureus (Sa) pI258 CadC (30, 46), Listeria monocytogenes (Lm) CadC (30), E. coli R773 (Ec) ArsR (59), S. aureus (Sa) CzrA (3), M. tuberculosis (Mtb) NmtR (20, 60), M. tuberculosis (Mtb) CmtR (22, 24, 25), M. tuberculosis (Mtb) KmtR (7), Xylella fastidiosa (Xf) BigR (27, 29). For Obr BxmR, the metals sensed by the α3N site are shaded green; ZnII is sensed by both α5 and α3N sites (this work); for ZiaR, both sites are apparently required to sense ZnII in vivo (52). Although the inducer identity of Xf BigR is not yet known (?), BigR contains conserved Met, Cys and Cys residues in the projected α1, α2 and α5 helices (27). See text for additional details.
