Abstract
Understanding the infant host response to measles vaccination is important because of their increased mortality from measles and the need to provide effective protection during the first year of life. Measles-specific T- and B-cell responses are lower in infants after measles vaccination than in adults. To define potential mechanisms, we investigated age-related differences in measles-specific T-cell proliferation, CD40-L expression, and IFN-γ production after measles immunization, and the effects of rhIL-12 and rhIL-15 on these responses. Measles-specific T-cell proliferation and mean IFN-γ release from infant PBMCs were significantly lower when compared with responses of vaccinated children and adults. Infant responses increased to ranges observed in children and adults when both rhIL-12 and rhIL-15 were added to PBMC cultures. Furthermore, a significant rise in T-cell proliferation and IFN-γ release was observed when infant PBMCs were stimulated with measles antigen in the presence of rhIL-12 and rhIL-15 compared to measles antigen alone. CD40-L expression by infant and adult T cells stimulated with measles antigen was comparable, but fewer infant CD40-L+ T cells expressed IFN-γ. These observations suggest that lower measles-specific T-cell immune responses elicited by measles vaccine in infants may be due to diminished levels of key cytokines.
Introduction
Measles remains a substantial cause of global childhood mortality, with highest rates in developing nations (10), and measles is an outbreak threat in the majority of developed countries (7,9). In 2004, the estimated global measles mortality was 454,000; of these deaths, 410,000 were children under the age of 5 y (10). Measles-specific mortality is highest among children <12 months of age (1,13), but the etiology of the excess mortality is unknown. One hypothesis is that the immunosuppressive effects of measles virus are more pronounced in the youngest age group, increasing their susceptibility to secondary infections (27,28). Measles infection produces abnormalities of nearly all the compartments of the human immune system, but prolonged suppression is marked by immunologic patterns consistent with a diminished T-helper type 1 (Th-1) response (3,16,24–26,36,46,52,60,61,65). One mechanism that unifies the observed measles-specific immune abnormalities is the downregulation of IL-12 by measles virus (3,36,50). In addition to and perhaps in combination with a greater susceptibility to the virus-specific immunosuppressive effects, the immature immune system in infants may limit the virus-specific host response further, thereby increasing the risk of measles mortality in infants.
Ideally, it would be advantageous to immunize infants less than 1 y of age to provide protection at the time of greatest susceptibility to adverse outcomes, but success to date has been limited (39,44,49). Thus far, our studies using measles vaccine as a model of T-cell-dependent antiviral immunity indicate that healthy infants have relative limitations in, but not absence of, the immune response to measles vaccine that persist during the second 6 mo of life. Infant responses include reduced measles-specific neutralizing antibody titers and T-cell proliferation, as well as lower IFN-γ and IL-12 production compared with vaccinated adults (18–20). Our studies evaluating other cytokines showed no IL-4 production and no age-related difference in IL-10 release in infant memory responses to measles vaccine. Therefore, the relative limitations observed in young infant immune responses to measles vaccine appear to be restricted to the Th-1 pathway (18).
Interleukin-12 is a crucial early stimulus for Th-1 clonal expansion since it stimulates resting and activated T cells, inducing the production of IFN-γ by T cells, activating NK cells, and inhibiting IL-4 production (12,32,40,55). In our previous studies focusing on the relative T-cell limitations in the responses of infants to measles antigen, we demonstrated that the addition of exogenous IL-12 to infant T-cell cultures did not enhance T-cell function to adult levels (19). This suggests that the observed deficiency in the measles-specific T-cell responses in infants is a more complex limitation of infant immune cells, likely to include other key cytokines. Like IL-12, IL-15 is an important cytokine made by activated antigen-presenting cells (APCs), which has both T- and B-cell stimulatory activity (30,43). Decreased IL-15, along with low IL-12 production, would decrease humoral immune responses as well as adaptive T-cell immunity, both of which are seen in infants immunized with measles vaccine. The accumulating evidence is that healthy older infants and young children have relative deficiencies in T-cell and APC function compared to adults (51,58), and while these do restrict immune responses in these populations, younger infants may have more profound deficiencies that do interfere with adaptive immunity. Observations from studies of antiviral immunity in newborns have shown, among other defects, limited T-cell proliferation and production of key cytokines (37,63,64). Relative deficiencies in antiviral T-cell immunity have been shown in newborns congenitally infected with cytomegalovirus (CMV) (21–23,31), and prolonged viral shedding in these individuals indicates that sufficient antiviral immunity is not obtained for months to years into childhood (29). Studies with other antigens, such as BCG, HIV, and influenza have also indicated a maturational T-cell response to these antigens during infancy (34,41,62).
Mechanisms explaining the relative limitations of infant immune responses to measles vaccine are likely to be multifactorial and are largely unstudied to date. Deficiencies in T-cell activation after measles immunization may be a result of diminished expression of CD40-ligand (CD40-L) on activated T cells in infants. CD40-L is a crucial cytokine that is important for providing signals to the B cell (via its interaction with CD40), allowing for antibody production and isotype switching (54,59). CD40-L is also a key APC activating factor, resulting in secretion of IL-12 (47,56), and plays an important role in priming of T-cell production of IFN-γ (47,56,59). Neonatal cells have been shown to have reduced expression of CD40-L (14,17,35,48), but when levels reach optimal function is unknown. Deficiencies may still be present in older infants, accounting for depressed cell-mediated immunity and decreased ability to develop humoral immune responses as has been reported after measles immunization in infants (18,19).
The purpose of this study was to compare the virus-specific T-cell responses in infants with adults who had vaccine-induced measles immunity. Our hypothesis was that infants might have a relatively limited Th-1-like response to measles antigen compared to adults, which is impacted by diminished levels of cytokines needed for effective adaptive immunity. We chose to study the antigen-specific responses in infants as opposed to mitogen stimulation, as this is more representative of what an infant's immune system will encounter and will give the most clinically relevant information. Building upon our previous work with IL-12, we expanded our evaluation to the effect of both IL-15 and IL-12, key cytokines released by activated DCs, on enhancing measles-specific CD4+ T-cell proliferation and IFN-γ production when infant and adult PBMCs were incubated with measles antigen. In addition, we explored the hypothesis that decreased IL-12 expression and IFN-γ production may be a result of limited CD40-L expression by activated infant T cells, restricting T-cell interactions with APCs and B cells, and resulting in diminished adaptive T- and B-cell immunity. In addition to being of public health interest to improve the effectiveness of measles vaccination of young infants against measles before 12 mo of age, the results of these analyses of infant PBMC responses to measles antigen may apply more broadly to an infant's ability to respond to viral antigens as is supported by our clinical observations with other viruses (31,37,63).
Methods
Populations
Subjects included 115 infants receiving well child care in Palo Alto, California, who received their primary measles vaccination at 6 (n = 33), 9 (n = 35), or 12 (n = 47) mo of age. Blood samples were taken before, 12 wk after monovalent measles vaccine given at 6 or 9 months of age, or 24 weeks after MMR administered to the 12-month-old infants. Ten children (aged 5–11 y) and 15 adults (aged 20–49 y) who had received two doses of MMR (the children according to the routine schedule of 12 mo and 5 y, and adults in childhood) were also evaluated with one blood sample. Not all assays were performed on all samples.
Exclusion criteria for the infant participants were gestation <36 wk, birth weight <2500 g, and for all subjects, acute or chronic illness. No measles cases were identified in our area during the study period of 1994–2002.
The study was approved by the Stanford University Committee for the Protection of Human Subjects and the Institutional Review Board of the Palo Alto Medical Foundation; written consent was obtained from parents or guardians.
Vaccines
Six- and 9-month-old infants received Measles Virus Vaccine Live (Attenuvax; Merck, Inc., West Point, PA) and 12-month-old infants received one dose of MMR-II.
T-cell proliferation assay
Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by Ficoll-Hypaque gradient and added to 96-well microtiter plates at concentrations of 3.0 × 105/well in RPMI 1640 (Gibco, Inc., Gaithersburg, MD), and 10% normal human sera (Sigma, Inc., St. Louis, MO). Measles antigen, prepared from lysates of Vero cells inoculated with Attenuvax measles vaccine (More Attenuated Enders' Strain; Merck, Inc., West Point, PA), or an uninfected cell control (both with final concentration 320 μg/mL) were added at dilutions of 1:16 and 1:32 to triplicate wells for testing infant PBMCs and in quadruplicate wells for adults. Adult PBMCs were also incubated with antigen and control at a 1:64 dilution. Preliminary studies were performed with multiple measles antigen dilutions (range 1:16–1:512); dilutions of 1:16 and 1:32 stimulated T cells from all positive donors. Recombinant human IL-12 (rhIL-12), with a bioactivity of 7.7 × 107 U/mL, generously provided by Genetics Institute, Inc. (Cambridge, MA), and recombinant human IL-15 (rhIL-15) generously provided by Dr. Elaine Thomas courtesy of Immunex, were added alone or in combination to PBMCs stimulated with measles antigen or uninfected cell control at optimal concentration determined in pilot assays. Preliminary studies were performed using multiple concentrations of both rhIL-12 and rhIL-15, based on published experience and consultation with the manufacturer; additional concentrations above and below these standards were also tested. The optimal concentration for rhIL-12 was determined to be 5 U/mL (0.29 ng/mL), and for rhIL-15 0.25 ng/mL and 2.5 ng/mL, which were used in all further experiments. The concentration yielding the highest results was used for statistical analysis. T-cell proliferation was measured by adding tritiated-thymidine after 5 d (1.25 μCi/mL) for 6–18 h. T-cell proliferation was calculated by using the mean counts per minute (cpm) in measles antigen-stimulated wells minus the mean cpm in control wells. The highest cpm from either concentration of measles antigen in the presence or absence of rhIL-12 and rhIL-15 was used for statistical analysis. The mean cpm in background wells were compared between infant groups to assure that no significant age-related differences existed that might influence results. PBMCs were also stimulated with phytohemagglutinin (PHA; .01 mg/mL; Difco, Inc., Detroit, MI), positive control, and phosphate-buffered saline (PBS), negative control (data not shown).
Cytokine production
Supernatants from PBMCs stimulated with measles antigen or uninfected cell control in the presence or absence of rhIL-12 (final concentration 5 U/mL) or rhIL-15 (final concentration 0.25 ng/mL and 2.5 ng/mL) each alone or in combination were collected from wells on days 5 and 7, and stored at −70°C. Preliminary studies showed peak production of IFN-γ between days 5 and 7 post-stimulation. Supernatants were tested for IFN-γ using the ELISA method from Biosource, Inc. (Camarillo, CA). Peak values from either concentration or day were used for statistical analysis. Sensitivities of cytokine detection were defined by reference standards in each assay.
Flow cytometry assay
Cell surface markers and intracellular cytokine staining
Aliquots of 200 μL of fresh, heparinized whole blood were co-stimulated with anti-CD28 and anti-CD49d monoclonal antibodies (BD Biosciences, San Jose, CA), followed by 16 μL, 24 μL, and 40 μL of measles antigen or Vero cell lysates as prepared above. Staphylococcus aureus enterotoxin B (final concentration of 0.5 mg/mL; Sigma-Aldrich) was used as a positive control, and PBS the negative control for each subject (data not shown). Samples were incubated for 6 h at 37°C, in 5% CO2, and brefeldin A (final concentration of 10 μg/mL; Sigma-Aldrich) was added for the final 4 h of the incubation period. After the incubation, 20 μL of 20 mM EDTA (Sigma) was added, followed by FACS Lysing Solution 1X (BD Biosciences). The samples were then centrifuged for 5 min and the supernatant was discarded and the cell pellet was resuspended in 10% dimethyl sulfoxide in BSA (Sigma). Samples were frozen at −80°C and then processed/stained within 2 wk. Frozen samples were thawed, cells permeablized with FACS permeabilizing solution (BD Biosciences), and stained in the dark for 30 min with the following mixture of fluorochrome-conjugated monoclonal antibodies (all BD Biosciences): CD4-PerCP-Cy5.5, IFN-γ-FITC, and CD40L-APC. The stained cells were washed with wash buffer and fixed with 1% paraformaldehyde in PBS.
Flow cytometric analysis
Samples were analyzed using a FACS Calibur flow cytometer (BD Immunocytometry Systems). The lymphocyte population was gated using forward versus side scatter and setting the acquisition gate to acquire CD4+ T cells. Approximately 50,000 events were collected for each sample, and populations were identified by gating on CD4+CD40L+IFN-γ+ cells. Acquisition and analysis were performed with Cell-quest Pro software (BD Biosciences). Data were analyzed as quadrant percentage of positive cells that were CD4+ and CD40-L-positive and then CD4+CD40L+IFN-γ+. Total lymphocyte counts were not accessed as part of this study, but many previous studies have documented that the absolute CD4+ T-cell count is on average twofold higher in the same amount of blood from infants compared with adult counts (15,33). To account for this difference we modified the frequency of values in infants by multiplying by a factor of two as previously reported (57).
Statistics
Responses were compared by Student's paired or unpaired t-test. T-cell proliferation is reported as mean counts per minute (cpm) and standard error (SE), and as stimulation index (SI) and SE. IFN-γ is reported as mean concentration (pg/mL) and SE. Flow cytometry data are presented as percentage of positive events in the antigen-stimulated samples minus the percentage of events in the Vero cell controls. p < 0.05 was considered statistically significant.
Results
Measles-specific T-cell proliferation responses in the presence and absence of rhIL-12 and rhIL-15
Lymphocyte proliferation was measured in five infants (6–12 mo of age) and five adults (20–49 y of age), comparing stimulation of PBMCs with measles antigen alone or in the presence of rhIL-12 and rhIL-15, each alone or in combination. Since not all infant samples yielded enough PBMCs to test the stimulation index after stimulation with measles antigen and rhIL-15, statistics were not analyzed using the results from this condition.
Infant T-cell responses
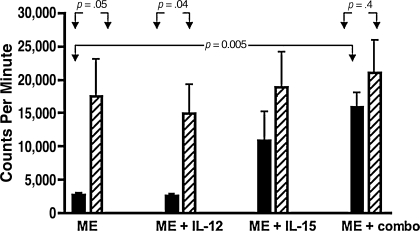
Overall, the T-cell proliferation response in infant PBMC cultures was equivalent under all conditions, except when rhIL-12 in combination with rhIL-15 was added to cultures with measles antigen, which resulted in significantly higher cpm ± SE. The mean cpm ± SE after measles antigen stimulation of infant PBMCs was 2780 (±307), which was equivalent to the mean cpm ± SE of 2632 (±280) with measles in the presence of rhIL-12. These results were significantly lower when compared with the mean cpm ± SE of 15,919 (±2145) observed after stimulation with measles antigen and both rhIL-12 and rhIL15 (p = .005) (Fig. 1).
FIG. 1.
T-cell proliferation in infants after measles immunization compared with vaccinated adults. Shown are the mean counts per minute (cpm) and standard error (SE) from measles-stimulated PBMCs in infants (solid bars) after measles immunization (ME), and vaccinated adults (hatched bars) in the presence and absence of human recombinant interleukin-12 or interleukin-15 or both. Reported cpm were calculated as cpm in antigen-stimulated wells minus the cpm in control wells. Blood samples were collected 12 or 24 wk after measles immunization in infants who were 6, 9, or 12 mo of age at the time of immunization and in adults aged 20–49 y.
Adult T-cell responses
The pattern observed with infant responses was not seen with PBMCs from adults. The mean cpm ± SE were similar under all conditions tested and are represented in Fig. 1.
Infant T-cell responses compared with adult responses
Infant T-cell responses were lower than those seen in adults when comparing cpm ± SE in the presence of measles antigen alone, and measles and rhIL-12 (all p < .05). This is in contrast to the equivalent mean cpm ± SE when infant and adult PBMCs were stimulated with measles antigen and both rhIL-12 and rhIL-15, respectively (p = .4) (Fig. 1).
Measles-specific IFN-γ production in the presence and absence of rhIL-12 and rhIL-15
Infant IFN-γ production
To assess baseline responses in measles-naïve infants, IFN-γ release in PBMC cultures after stimulation with measles antigen alone and in the presence of rhIL-12 or rhIL-15 alone or in combination was measured in 16 infants. The infants were 6 mo (n = 6), 9 mo (n = 5), or 12 mo (n = 5) old, and were tested just before measles immunization (data not shown). No age-related responses were noted at baseline. There was a measles-specific response detected when PBMCs from these infants were tested after measles immunization and compared with responses at baseline.
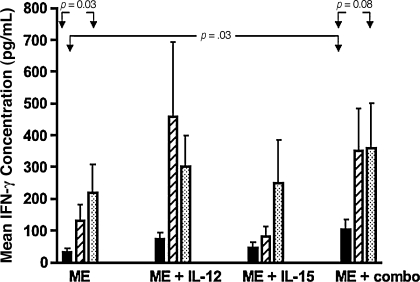
The mean IFN-γ concentrations (±SE) produced by infant PBMCs after measles immunization were 33 (±11), 72 (±22), 44 (±19), and 104 (±32) with measles antigen, measles antigen and rhIL-12, measles antigen and rhIL-15 and measles antigen and rhIL-12 and rhIL-15, respectively (Fig. 2). There was a significant rise in IFN-γ concentration when comparing the mean IFN-γ concentration with measles antigen stimulation alone to the mean concentrations in the presence of measles antigen and both rhIL-12 and rhIL-15 (p = .03), but not when each cytokine was added alone to measles-antigen simulated PBMCs.
FIG. 2.
Mean IFN-γ concentration from stimulated PBMCs in infants after measles immunization compared with vaccinated children and adults. Shown is the mean IFN-γ concentration (pg/mL) and standard error (SE) released from measles-stimulated PBMCs in infants (solid bars) after measles immunization (ME), and in vaccinated children (striped bars) and adults (dotted bars) in the presence and absence of rhIL-12 or rhIL-15 or both. Infants were 6, 9, or 12 mo of age at the time of immunization, and samples were collected 12–24 wk after immunization.
IFN-γ production by PBMCs from children and adults
While some increase in IFN-γ release was observed when PBMCs of older children (n = 10) and adults (n = 15) were stimulated with measles and rhIL-12 and rhIL-15, this increase was not significant, in contrast to responses observed in infants. The mean IFN-γ concentrations (±SE) released from PBMCs from older children and adults are shown in Fig. 2.
Infant IFN-γ production responses compared with children and adult responses
The mean IFN-γ release in infant PBMC cultures was significantly lower when compared with results in PBMC cultures from children and adults after measles stimulation (p = .03 and p = .05, respectively). Responses increased to levels equivalent to those of adult PBMCs in the presence of measles and both rhIL-12 and rhIL-15 (p = .08), and not each cytokine alone.
Measles-specific CD40-L production by CD4+ T cells
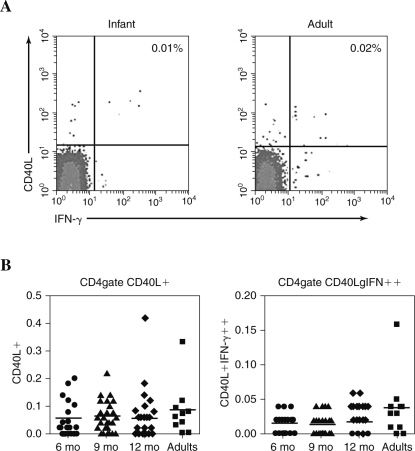
The mean frequencies of measles-specific T cells expressing CD40-L were evaluated in 94 children who were vaccinated against measles at 6 (n = 26), 9 (n = 29) and 12 (n = 39) mo of age, and in 14 adults (representative plots are shown in Fig. 3A). The mean percentages were equivalent among infant groups and when compared to those of adults. The mean frequencies were 0.05%, 0.06%, 0.05%, and 0.08%, in infants at 6, 9, and 12 mo of age, and adults, respectively (Fig. 3B). These evaluations were also performed using the mean number of events to distinguish responses without the complication of differences in cell populations, and results were the same as when comparing percentages.
FIG. 3.
Flow cytometric analysis of measles-specific CD4+ T-cell responses in vaccinated infants compared with adults. (A) Shown are representative results of measles-specific CD4+ T cells that express CD40-L (right and left top panels) and IFN-γ (right top and bottom panels) from a 9-mo-old infant (left histogram) and an adult (right histogram). The numbers in each histogram represent the percentage of measles-specific CD4+CD40-L+ T cells that also express IFN-γ (top right panel) from 50,000 CD4+ T cells. The infant was immunized with Attenuvax at 9 mo of age and a blood sample collected 12 wk after immunization. (B) Shown are the individual percentages and mean percentage of measles-specific CD4+ T cells that express CD40-L (left panel) and both CD40-L and IFN-γ (right panel) in infants vaccinated with measles at 6 (circles), 9 (triangles), 12 (diamonds) months of age, and vaccinated adults (squares). Infants were 6, 9, or 12 mo of age at the time of immunization, and samples were collected 12–24 wk after immunization.
Measles-specific CD40-L production and IFN-γ expression by CD4+ T cells
A significant rise in the measles-specific production of IFN-γ was observed from CD40-L-positive CD4+ T cells when responses after vaccination were compared to those before measles immunization in the three infant groups (data not shown; all p < .05). Mean percentages after measles vaccination were 0.01%, 0.01%, and 0.02%, in infants 6 (n = 25), 9 (n = 27), and 12 (n = 39) months of age, respectively. These responses did not show any significant age-related differences (Fig. 3B). The mean percentage for measles-specific double positive CD4+ T cells that were both CD40-L and IFN-γ positive was significantly lower in all infant groups compared with the mean percentage of 0.04% observed in adult CD4+CD40-L+IFN-γ+ T cells (p ≤ .04) (Fig. 3B).
Discussion
Despite the introduction of an effective vaccine over four decades ago, measles accounts for 37% of vaccine-preventable childhood mortality (11,45), most of which occurs during the first year of life (2,8). Increased mortality rates in infants less than 12 mo of age supports the need for earlier protection against measles disease through vaccination. Obstacles to early immunization include the limitations of the developing immune system. We have reported relative deficiencies of adaptive T- and B-cell immunity to measles in infants given measles vaccine compared with responses in adults. These include limited T-cell proliferation, diminished IFN-γ production by stimulated T cells, and reduced augmentation of IFN-γ release by infant T cells in the presence of IL-12 (18–20).
The current data reveal a correction of the limited T-cell responses to measles antigen in infants when exogenous IL-12 and IL-15 were added to PBMC cultures along with measles antigen. When infant T cells were incubated with measles antigen alone, measles-specific T-cell proliferation and IFN-γ production from stimulated PBMCs was low compared with older children and adults, but responses increased to equivalent levels in the presence of IL-12 and IL-15 in combination. Furthermore, significant increases were observed when infant T-cell proliferation and IFN-γ responses to measles antigen alone were compared with responses in the presence of IL-12 and IL-15 in combination, supporting the capacity of these cytokines to correct the deficiency. This effect was not observed for IFN-γ responses for children and adults, and adult T-cell proliferative responses, which were robust in the presence of measles antigen alone, and were not significantly increased further by the addition of exogenous cytokines. This suggests that IL-12 and IL-15 concentrations produced by PBMCs from adults and older children were sufficient to induce and amplify measles T-cell responses maximally.
Since IL-12 and IL-15 are crucial cytokines produced by professional APCs or dendritic cells (DCs), these data suggest a role of the APCs in the lower responses of infant T cells to stimulation by measles antigen. The relative deficiencies that have been observed could be explained by a diminished capacity of infant APCs to be stimulated for the final stages of maturation and produce the cytokines necessary for augmentation of the memory T-cell response.
Mechanistically, reduced levels of CD40-L could explain incomplete DC activation and diminished production of cytokines. In this study, we examined the antigen-specific induction of CD40-L surface expression as a potential mechanism explaining the reduced T- and B-cell responses to measles vaccination that were previously reported (18–20), and as a potential mechanism for diminished DC activation. Previous studies indicate that CD40-L upregulation in response to mitogen stimulation is diminished in newborns (14,17,35,48). Our data show that the CD40-L expression from infant CD4+ T cells was comparable to the expression seen on adult CD4+ T cells in response to measles antigen stimulation. However, despite equivalent activation, infant CD4+ T cells expressing CD40-L were significantly less likely to produce IFN-γ when compared with the same subset of activated adult CD4+ T cells. Thus, subsequent differentiation of CD4+ T cells into effector cells with expression of IFN-γ in response to measles antigen was less robust in infants.
Our previous data have shown that measles antibody responses after measles immunization are diminished in 6-mo-old infants compared with older infants, even in the absence of passively acquired maternal antibodies to measles (18), but examination of the immunoglobulin isotype revealed that measles IgG antibodies are produced. This suggests that the interaction between CD40-L on activated T cells with CD40 on B cells was intact, allowing for isotype switching, which is a crucial role for CD40-L (5). The findings in the current study suggest that upregulation of CD40-L expression on activated T cells is intact after measles antigen stimulation, so that a deficiency in this pathway would not be expected to be responsible for the limitations seen in infant immune responses to measles vaccine.
It has been shown that after the initial interaction between activated T cells and APCs via CD40-L-CD40 binding, a maturation that produces a professional APC must occur. This mature APC can then prime T cells, allowing the T cell to complete the immune response via interactions with other cell types (6,38). Based on the current findings, we speculate that the additional feedback from the professional APC to the CD4+ T cell, through key cytokines produced by DCs or monocytes, including IL-12 and IL-15, was lacking, while initial activation of the infant CD4+ T cell was intact. It has been shown that both IL-12 and IL-15 are important for the enhancement of IFN-γ expression from T cells, allowing for the clonal expansion of activated T cells that is necessary for effective adaptive immunity (4,6,42,53). IL-12 is crucial for T-cell immunity and IL-15 has been shown to have both T- and B-cell stimulatory effects (43,53,55). Diminished production of IL-12 and IL-15 from APCs would be expected to cause restricted T-cell proliferation by limiting upregulation of co-stimulatory molecules, and thereby reducing the APC-dependent IFN-γ release that is needed for the final steps in the adaptive immune response and T-cell expansion (43,55). In addition, IL-15 is critical for upregulating CD40 expression on monocytes, further enhancing IFN-γ levels by promoting additional CD40-L-CD40 interactions (4).
The acquisition of antiviral immunity is complex, but clinical and laboratory data support limitations of the immune response in infants and young children to several viral pathogens. The findings in this study suggest a possible mechanism for these observations. Our data suggest that early T-cell activation, marked by the expression of CD40-L in response to measles antigen, is functional as early as 6 mo of age, but that the later stages of T-cell and APC activation and maturation are reduced compared with older children and adults. While most likely multifactorial, the correction of the limited T-cell responses in vitro by the addition of IL-12 and IL-15 to infant PBMCs focuses attention on the role of the APC, the source of these key cytokines, as a factor in the diminished virus-specific cellular immunity seen in infants. Future evaluations determining the cellular mechanism responsible for restricted APC function are necessary to fully understand these findings.
Conclusion
The importance of immunizing infants less than 1 y of age against measles is supported by the increased mortality in this age group. It is important to understand the limitations of the developing immune system in order to develop effective vaccines and appropriate vaccine dose regimens for use in infancy. To our knowledge, this is the first study that has highlighted the potential role of reduced function of antigen-presenting cells in the limited virus-specific immune response of infants beyond the neonatal period.
Acknowledgments
We are indebted to the families, pediatricians, nursing staff, and laboratory staff of the Palo Alto Medical Foundation for their assistance with this study. This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI37127), Bethesda, Maryland.
References
- 1.Aaby P. Andersen M. Knudsen K. Excess mortality after early exposure to measles. Int J Epidemiol. 1993;22:156–162. doi: 10.1093/ije/22.1.156. [DOI] [PubMed] [Google Scholar]
- 2.P Aaby. J Clements. V Orinda. Presented at the Expanded Programme on Immunization; Geneva. 1991. [Google Scholar]
- 3.Atabani SF. Byrnes AA. Jaye A. Kidd IM. Magnusen AF. Whittle H. Karp CK. Natural measles causes prolonged suppression of interleukin-12 production. J Infect Dis. 2001;184:1–9. doi: 10.1086/321009. [DOI] [PubMed] [Google Scholar]
- 4.Avice MN. Demeure CE. Delespesse G. Rubio M. Armant M. Sarfati M. IL-15 promotes IL-12 production by human monocytes via T cell-dependent contact and may contribute to IL-12-mediated IFN-gamma secretion by CD4+ T cells in the absence of TCR ligation. J Immunol. 1998;161:3408–3415. [PubMed] [Google Scholar]
- 5.Banchereau J. Bazan F. Blanchard D, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J. Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. Prevention: Epidemiology of measles—United States, 2001–2003. MMWR Morb Mortal Wkly Rep. 2004;53:713–716. [PubMed] [Google Scholar]
- 8.Centers for Disease Control, Prevention. Global measles control and regional elimination, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48:1124–1130. [PubMed] [Google Scholar]
- 9.Centers for Disease Control, Prevention: Measles—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1348–1351. [PubMed] [Google Scholar]
- 10.Centers for Disease Control, Prevention: Progress in reducing global measles deaths, 1999–2004. MMWR Morb Mortal Wkly Rep. 2006;55:247–249. [PubMed] [Google Scholar]
- 11.Centers for Disease Control, Prevention: Update: Global measles control and mortality reduction—worldwide, 1991–2001. MMWR Morb Mortal Wkly Rep. 2003;52:471–475. [PubMed] [Google Scholar]
- 12.Chehimi J. Trinchieri G. Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol. 1994;14:149–161. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 13.Clements CJ. Cutts FT. The epidemiology of measles: thirty years of vaccination. Curr Top Microbiol Immunol. 1995;191:13–33. doi: 10.1007/978-3-642-78621-1_2. [DOI] [PubMed] [Google Scholar]
- 14.Durandy A. De Saint Basile G. Lisowska-Grospierre B, et al. Undetectable CD40 ligand expression on T cells and low B cell responses to CD40 binding agonists in human newborns. J Immunol. 1995;154:1560–1568. [PubMed] [Google Scholar]
- 15.Erkeller-Yuksel FM. Deneys V. Yuksel B, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr. 1992;120:216–222. doi: 10.1016/s0022-3476(05)80430-5. [DOI] [PubMed] [Google Scholar]
- 16.Fugier-Vivier I. Servet-Delprat C. Rivailler P. Rissoan MC. Liu YJ. Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuleihan R. Ahern D. Geha RS. Decreased expression of the ligand for CD40 in newborn lymphocytes. Eur J Immunol. 1994;24:1925–1928. doi: 10.1002/eji.1830240832. [DOI] [PubMed] [Google Scholar]
- 18.Gans H. Arvin A. Galinus J. Logan L. DeHovitz R. Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA. 1998;280:527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 19.Gans H. Maldonado Y. Yasukawa L. Beeler J. Rinki M. DeHovitz R. Arvin A. Interleukin 12, interferon-g and T cell proliferation to measles in immunized infants. J Immunol. 1999;162:5569–5575. [PubMed] [Google Scholar]
- 20.Gans H. Yasukawa L. Alderson A, et al. Humoral and cell-mediated immune responses to an early two-dose measles vaccination regimen in the United States. J Infect Dis. 2004;190:83–90. doi: 10.1086/421032. [DOI] [PubMed] [Google Scholar]
- 21.Gehrz RC. Linner KM. Christianson WR. Ohm AE. Balfour HH., Jr. Cytomegalovirus infection in infancy: virological and immunological studies. Clin Exp Immunol. 1982;47:27–33. [PMC free article] [PubMed] [Google Scholar]
- 22.Gehrz RC. Marker SC. Balfour HH., Jr. Specific cell-mediated immune defect in congenital cytomegalovirus infection. Lancet. 1977;1:811–812. doi: 10.1016/s0140-6736(77)93007-0. [DOI] [PubMed] [Google Scholar]
- 23.Gehrz RC. Peterson ES. Liu YN. Immune mechanisms in congenital cytomegalovirus infection: activation of CMV-specific T helper cells (CMV-Th) by exogenous IL-2. Clin Exp Immunol. 1988;74:333–338. [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin DE. Immune responses during measles virus infection. Curr Top Microbiol Immunol. 1995;191:117–134. doi: 10.1007/978-3-642-78621-1_8. [DOI] [PubMed] [Google Scholar]
- 25.Griffin DE. Moench TR. Johnson RT. Lindo de Soriano I. Vaisberg A. Peripheral blood mononuclear cells during natural measles virus infection: cell surface phenotypes and evidence for activation. Clin Immunol Immunopathol. 1986;40:305–312. doi: 10.1016/0090-1229(86)90035-8. [DOI] [PubMed] [Google Scholar]
- 26.Griffin DE. Ward BJ. Differential CD4 T cell activation in measles. J Infect Dis. 1993;168:275–281. doi: 10.1093/infdis/168.2.275. [DOI] [PubMed] [Google Scholar]
- 27.Griffin DE. Ward BJ. Esolen LM. Pathogenesis of measles virus infection: an hypothesis for altered immune responses. J Infect Dis. 1994;170(Suppl 1):S24–S31. doi: 10.1093/infdis/170.supplement_1.s24. [DOI] [PubMed] [Google Scholar]
- 28.Griffin DE. Ward BJ. Jauregui E. Johnson RT. Vaisberg A. Immune activation in measles. N Engl J Med. 1989;320:1667–1672. doi: 10.1056/NEJM198906223202506. [DOI] [PubMed] [Google Scholar]
- 29.Hanshaw JB. Congenital cytomegalovirus infection: a fifteen year perspective. J Infect Dis. 1971;123:555–561. doi: 10.1093/infdis/123.5.555. [DOI] [PubMed] [Google Scholar]
- 30.Hasan MS. Kallas EG. Thomas EK. Looney J. Campbell M. Evans TG. Effects of interleukin-15 on in vitro human T cell proliferation and activation. J Interferon Cytokine Res. 2000;20:119–123. doi: 10.1089/107999000312513. [DOI] [PubMed] [Google Scholar]
- 31.Hassan J. Dooley S. Hall W. Immunological response to cytomegalovirus in congenitally infected neonates. Clin Exp Immunol. 2007;147:465–471. doi: 10.1111/j.1365-2249.2007.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heufler C. Koch F. Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 33.Hulstaert F. Hannet I. Deneys V. Munhyeshuli V. Reichert T. De Bruyere M. Strauss K. Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994;70:152–158. doi: 10.1006/clin.1994.1023. [DOI] [PubMed] [Google Scholar]
- 34.Ildirim I. Sapan N. Cavusoglu B. Comparison of BCG vaccination at birth and at third month of life. Arch Dis Child. 1992;67:80–82. doi: 10.1136/adc.67.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jullien P. Cron RQ. Dabbagh K, et al. Decreased CD154 expression by neonatal CD4+ T cells is due to limitations in both proximal and distal events of T cell activation. Int Immunol. 2003;15:1461–1472. doi: 10.1093/intimm/dxg145. [DOI] [PubMed] [Google Scholar]
- 36.Karp CL. Wysocka M. Wahl LM, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 37.Lewis D. Wilson C. Developmental Immunology Role of the Host Defenses in Neonatal Susceptibility to Infection. 4th. >Saunders; Philadelphia: 1995. [Google Scholar]
- 38.Mackey MF. Barth RJ., Jr. Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 39.Markowitz LE. Sepulveda J. Diaz-Ortega JL, et al. Immunization of six-month-old infants with different doses of Edmonston-Zagreb and Schwarz measles vaccines [published erratum appears in N Engl J Med 1990;322:863] N Engl J Med. 1990;322:580–587. doi: 10.1056/NEJM199003013220903. [DOI] [PubMed] [Google Scholar]
- 40.Marshall JD. Secrist H. DeKruyff RH. Wolf SF. Umetsu DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- 41.Mbawuike IN. Piedra PA. Cate TR. Couch RB. Cytotoxic T lymphocyte responses of infants after natural infection or immunization with live cold-recombinant or inactivated influenza A virus vaccine. J Med Virol. 1996;50:105–111. doi: 10.1002/(SICI)1096-9071(199610)50:2<105::AID-JMV1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.McDyer JF. Li Z. John S. Yu X. Wu CY. Ragheb JA. IL-2 receptor blockade inhibits late, but not early, IFN-gamma and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and -independent pathways of IFN-gamma production. J Immunol. 2002;169:2736–2746. doi: 10.4049/jimmunol.169.5.2736. [DOI] [PubMed] [Google Scholar]
- 43.Mori A. Suko M. Kaminuma O, et al. IL-15 promotes cytokine production of human T helper cells. J Immunol. 1996;156:2400–2405. [PubMed] [Google Scholar]
- 44.Murphy MD. Brunell PA. Lievens AW. Shehab ZM. Effect of early immunization on antibody response to reimmunization with measles vaccine as demonstrated by enzyme-linked immunosorbent assay (ELISA) Pediatrics. 1984;74:90–93. [PubMed] [Google Scholar]
- 45.Murray C. Lopez A. Mathers C. Stein C. The global burden of disease 2000 project: aims, methods, and data sources. Global Programme on Evidence for Health Policy. Geneva, WHO. 2001;11:10–49. [Google Scholar]
- 46.Naniche D. Oldstone MB. Generalized immunosuppression: how viruses undermine the immune response. Cell Mol Life Sci. 2000;57:1399–1407. doi: 10.1007/PL00000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 48.Nonoyama S. Penix LA. Edwards CP, et al. Diminished expression of CD40 ligand by activated neonatal T cells. J Clin Invest. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pabst HF. Spady DW. Carson MM. Krezolek MP. Barreto L. Wittes RC. Cell-mediated and antibody immune responses to AIK-C and Connaught monovalent measles vaccine given to 6 month old infants. Vaccine. 1999;17:1910–1918. doi: 10.1016/s0264-410x(98)00472-1. [DOI] [PubMed] [Google Scholar]
- 50.Polack FP. Hoffman SJ. Moss WJ. Griffin DE. Altered synthesis of interleukin-12 and type 1 and type 2 cytokines in rhesus macaques during measles and atypical measles. J Infect Dis. 2002;185:13–19. doi: 10.1086/338009. [DOI] [PubMed] [Google Scholar]
- 51.Prescott SL. Taylor A. King B. Dunstan J. Upham JW. Thornton CA. Holt PG. Neonatal interleukin-12 capacity is associated with variations in allergen-specific immune responses in the neonatal and postnatal periods. Clin Exp Allergy. 2003;33:566–572. doi: 10.1046/j.1365-2222.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 52.Schnorr JJ. Xanthakos S. Keikavoussi P. Kampgen E. ter Meulen V. Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skov S. Bonyhadi M. Odum N. Ledbetter JA. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J Immunol. 2000;164:3500–3505. doi: 10.4049/jimmunol.164.7.3500. [DOI] [PubMed] [Google Scholar]
- 54.Splawski JB. Lipsky PE. CD40-mediated regulation of human B-cell responses. Res Immunol. 1994;145:226–234. doi: 10.1016/s0923-2494(94)80189-4. discussion 244–249. [DOI] [PubMed] [Google Scholar]
- 55.Stern AS. Magram J. Presky DH. Interleukin-12 an integral cytokine in the immune response. Life Sci. 1996;58:639–654. doi: 10.1016/s0024-3205(96)80003-8. [DOI] [PubMed] [Google Scholar]
- 56.Stuber E. Strober W. Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu W. Chen S. Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 58.Upham JW. Lee PT. Holt BJ, et al. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002;70:6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Kooten C. Banchereau J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 60.Ward BJ. Griffin DE. Changes in cytokine production after measles virus vaccination: predominant production of IL-4 suggests induction of a Th2 response. Clin Immunol Immunopathol. 1993;67:171–177. doi: 10.1006/clin.1993.1061. [DOI] [PubMed] [Google Scholar]
- 61.Ward BJ. Johnson RT. Vaisberg A. Jauregui E. Griffin DE. Cytokine production in vitro and the lymphoproliferative defect of natural measles virus infection. Clin Immunol Immunopathol. 1991;61:236–248. doi: 10.1016/s0090-1229(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 62.Wasik TJ. Jagodzinski PP. Hyjek EM. Wustner J. Trinchieri G. Lischner HW. Kozbor D. Diminished HIV-specific CTL activity is associated with lower type 1 and enhanced type 2 responses to HIV-specific peptides during perinatal HIV infection. J Immunol. 1997;158:6029–6036. [PubMed] [Google Scholar]
- 63.Wilson C. Immunologic basis of increased susceptibility of the neonate to infection. J Pediatr. 1986;108:1–12. doi: 10.1016/s0022-3476(86)80761-2. [DOI] [PubMed] [Google Scholar]
- 64.Wilson CB. Westall J. Johnston L. Lewis DB. Dower SK. Alpert AR. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest. 1986;77:860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zweiman B. Pappagianis D. Maibach H. Hildreth EA. Effect of measles immunization on tuberculin hypersensitivity and in vitro lymphocyte reactivity. Int Arch Allergy. 1971;40:834–841. doi: 10.1159/000230466. [DOI] [PubMed] [Google Scholar]