Abstract
Rationale: Secondary bacterial infection following rhinovirus (RV) infection has been recognized in chronic obstructive pulmonary disease.
Objectives: We sought to understand mechanisms by which RV infection facilitates secondary bacterial infection.
Methods: Primary human airway epithelial cells grown at air–liquid interface and human bronchial epithelial (16HBE14o-) cells grown as polarized monolayers were infected apically with RV. Transmigration of bacteria (nontypeable Haemophilus influenzae and others) was assessed by colony counting and transmission electron microscopy. Transepithelial resistance (RT) was measured by using a voltmeter. The distribution of zona occludins (ZO)-1 was determined by immunohistochemistry and immunoblotting.
Measurements and Main Results: Epithelial cells infected with RV showed 2-log more bound bacteria than sham-infected cultures, and bacteria were recovered from the basolateral media of RV- but not sham-infected cells. Infection of polarized airway epithelial cell cultures with RV for 24 hours caused a significant decrease in RT without causing cell death or apoptosis. Ultraviolet-treated RV did not decrease RT, suggesting a requirement for viral replication. Reduced RT was associated with increased paracellular permeability, as determined by flux of fluorescein isothiocyanate (FITC)-inulin. Neutralizing antibodies to tumor necrosis factor (TNF)-α, IFN-γ and IL-1β reversed corresponding cytokine-induced reductions in RT but not that induced by RV, indicating that the RV effect is independent of these proinflammatory cytokines. Confocal microscopy and immunoblotting revealed the loss of ZO-1 from tight junction complexes in RV-infected cells. Intranasal inoculation of mice with RV1B also caused the loss of ZO-1 from the bronchial epithelium tight junctions in vivo.
Conclusions: RV facilitates binding, translocation, and persistence of bacteria by disrupting airway epithelial barrier function.
Keywords: COPD, exacerbation, Haemophilus influenzae, tight junction, ZO-1
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The mechanisms by which rhinovirus infection leads to secondary bacterial infection in patients with underlying respiratory diseases are not well understood.
What This Study Adds to the Field
Rhinovirus infection facilitates transmigration of bacteria across polarized airway epithelia by dissociating zona occludens-1 from the tight junction complex, thereby impairing barrier function of the epithelium.
The coinfection of viruses and bacteria have been recognized in several respiratory diseases including pneumonia, otitis media (1–3), chronic obstructive pulmonary disease (COPD) (4–7) and cystic fibrosis (CF) (8–11). Viruses predispose the host to bacterial infection by various mechanisms including destruction of the respiratory epithelium, modulation of innate defenses, and alteration of cell membranes, which in turn facilitates bacterial adherence. Influenza virus damages both ciliated and nonciliated respiratory epithelial cells, leading to necrosis of the tracheobronchial airway epithelium (12, 13). Respiratory syncytial virus (RSV) can also cause injury to ciliated cells, leading to ciliostasis, loss of cilia, and impairment of mucociliary clearance (14). Glycoproteins elaborated by influenza virus and RSV during replication can integrate into the host cell surface and serve as receptors for bacteria (15–17). Viruses can also expose or up-regulate the expression of native receptors for bacteria on the host cell surface, thereby increasing the bacterial adherence and colonization (18–21).
Rhinoviruses (RVs), members of the Picornaviridae family, are divided into two groups based on receptor utilization. The major group of RVs, greater than 90% of the serotypes, bind to intercellular adhesion molecule (ICAM)-1, whereas the remaining minor group serotypes bind to low density lipoprotein receptor and related proteins. RVs are single-stranded RNA viruses responsible for the majority of upper respiratory tract infections and their complications, including otitis media, sinusitis, and bronchitis. By a sensitive detection method (polymerase chain reaction), RV was detected in 25.6% of viral-induced acute otitis media (1), and up to one-half of all viral-associated COPD and CF exacerbations (4–7, 9). In each of these conditions, coinfection of RV and bacteria are thought to play an important pathogenetic role. Acute otitis media in children has been attributed to coinfections with RV or RSV and bacteria, and RV infection positively correlated with the presence of Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis (1, 22). In COPD patients, exacerbations associated with both RV and nontypeable H. influenzae had greater bacterial loads, serum interleukin (IL)-6 levels, symptom counts and falls in FEV1 than exacerbations without both pathogens (7).
Mechanisms by which viral infections facilitate bacterial growth or acquisition of new bacteria are not well understood. One of the mechanisms by which RV may promote bacterial infections is by increasing adherence to and/or invasion of epithelial cells by bacteria. In vitro studies have shown that RV increases the adherence of S. pnumoniae to bronchial epithelial cells by up-regulating the expression of platelet-activating factor receptor (19). RV pretreatment also promotes internalization of Staphylococcus aureus into normally nonpermissive A549 respiratory epithelial cells, in part through increased expression of intercellular adhesion molecule (ICAM)-1 (20). Taken together, these observations suggest that RV infection can promote opportunistic bacterial infections in the airways, although the underlying mechanisms are not well understood.
In addition to providing a physical barrier, the airway epithelium represents a dynamic system for innate host defense. Tight junctions located at the apicolateral borders of adjacent airway epithelial cells contribute significantly to epithelial barrier function. Tight junctions regulate the selective passage of ions and solutes through the paracellular space and prevent paracellular migration of pathogens and their products from lumen to interstitium. Thus, pertubation of the tight junction barrier function may increase paracellular permeability, facilitate translocation of pathogens and their soluble products, and expose basolateral receptors. We hypothesized that, in addition to upregulating receptors for bacteria, RV also promotes bacterial transmigration across the epithelial barrier. We found that infection of polarized epithelium with major and minor group RV promotes the paracellular migration of nontypeable H. influenzae (NTHi), an opportunistic pathogen in COPD patients, by disrupting epithelial barrier function. This effect appears to be specific to RV, as it was not observed with either RSV or influenza A virus.
Some of the results of these studies have been previously reported in the form of an abstract (23).
METHODS
See the online data supplement for additional details.
Virus
RV serotypes 16, 39, and 1B and tissue culture-adapted influenza virus A (H1N1) were purchased from American Type Culture Collection (ATCC, Manassas, VA). RV and influenza stocks were generated by infecting HeLa cells and MDCK cells, respectively. RSV was provided by N. Lukacs (University of Michigan, Ann Arbor, MI). HeLa supernatants from uninfected cells served as controls (sham infection).
Bacteria and Growth Conditions
NTHi isolates (6P5H, 5P19H1, 45P9H1) were obtained from COPD patients (from T. Murphy, University of Buffalo, Buffalo, NY). Pseudomonas aeruginosa strain PA01 is a laboratory isolate. S. aureus was purchased from ATCC. For infection assays, bacteria were subcultured on chocolate, nutrient, or brain–heart infusion medium, scraped off the plate, and suspended in cell culture medium.
Cell Culture
Human airway epithelial cells, obtained from tracheal trimmings of anonymous donor lungs at transplantation, were grown at air–liquid interface for mucociliary differentiation, as previously described (24). Use of donor lungs was reviewed by the Institutional Review Board of the University of Michigan. 16HBE14o- human bronchial epithelial cells (25) and Calu-3 lung adenocarcinoma cells (ATCC) were grown in collagen-coated Transwells (Corning Life Sciences, Lowell, MA).
Infection of Cell Cultures and Measurement of Transepithelial Resistance
Differentiated primary airway epithelial cells or polarized 16HBE14o- or Calu-3 cells were infected apically with RV, RSV, influenza A, or an equivalent volume of sham and incubated for 24 hours. RT was measured by EVOM voltmeter (World Precision Instruments, Sarasota, FL).
Paracellular Permeability
16HBE14o- or Calu-3 cells were infected with RV39 at a multiplicity of infection (MOI) of 1 for 24 hours and paracellular permeability of FITC-inulin (1 mg/ml) was determined (24, 26). In selected experiments, cultures were infected with bacteria and transmigration assessed by colony counting.
Nonidet P-40-soluble and P-40-insoluble Cell Extracts
After treatment, cells were rinsed with phosphate buffered saline (PBS) and incubated with NP-40 solubilization buffer (15 min on ice). NP-40-insoluble material was pelleted, the supernatant was saved, and Laemmli reducing buffer added to the pellet, which was then heated (10 min, 100°C).
Western Blot Analysis
NP-40-soluble and NP-40-insoluble fractions were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and proteins transferred to polyvinylidene difluoride membranes. Membranes were incubated with antibodies to zona occludins (ZO)-1 (BD Biosciences, San Jose, CA) or β-actin (Invitrogen, Carlsbad, CA).
Confocal Indirect Immunofluorescence
Cultures were fixed in methanol and incubated with antibody to ZO-1 (1 μg/ml) or antibody to heat-killed NTHi (from G. Krasan, University of Michigan, Ann Arbor, MI).
Transmission Electron Microscopy
Polarized monolayers of 16HBE14o- cells were infected with RV39 (MOI, 1). After 24 h, cells were superinfected with bacteria and fixed and processed for electron microscopy. Sections were viewed on a Philips CM100 at 60 kV.
Detection of Apoptosis
Polarized 16HBE14o- cells were treated with thapsigargin (2–5 μM, 24 h) and apoptotic, nonnecrotic cells detected by flow cytometry with FITC-conjugated annexin V and propidium iodide (Sigma-Aldrich, St. Louis, MO).
Inoculation of Mice
C57BL/6 mice were inoculated intransally with RV1B (TCID50, 5 × 107/mouse) or equal volume of sham, as previously described (27). After 24 hours, mice were killed and lungs were perfused with ethylenediamine tetraacetic acid (EDTA), inflated and fixed in formalin, and embedded in paraffin.
RESULTS
RV Increases Bacterial Binding to and Transmigration across Well-differentiated Airway Epithelial Cells
Normal primary human airway epithelial cells, grown at air–liquid interface, polarize, form tight junctions, and differentiate into a mucociliary phenotype (Figures 1A and 1B). Differentiated cell cultures were infected apically with RV39 or RV1B (MOI of 1) or equivalent volume of sham, and incubated for 24 hours. NTHi (isolate 6P5H) were then added to the apical surface and incubated for another 24 hours. An aliquot of basolateral medium was plated to determine the number of bacteria transmigrating across the epithelium. To determine the number of bound bacteria, cells were washed to remove unassociated bacteria, lysed, and serial dilutions of lysates were plated. Epithelial cells infected with RV showed 2 log greater bound bacteria than the sham-infected or media-treated cell cultures (Table 1). In addition, bacteria were recovered from the basolateral media of RV-infected but not from sham-infected or medium-treated cell cultures. These results indicate that RV not only increases bacterial binding to epithelial cells but also facilitates bacterial transmigration across differentiated airway epithelia.
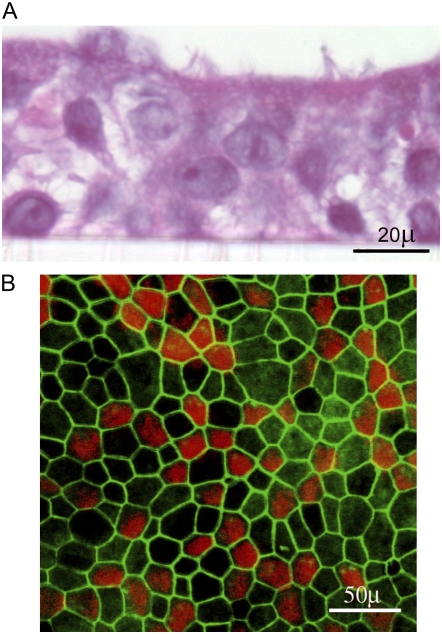
Figure 1.
Morphologic features of primary airway epithelial cells differentiated into mucociliary phenotype. (A) Primary airway epithelial cells were grown in transwells at an air/liquid interface. Cells were fixed in buffered formalin, embedded in agarose-paraffin, and sections were stained with hematoxilin and eosin. (B) Primary airway epithelial cells grown at air/liquid interface were fixed in methanol and stained with zona occludins ZO-1 (green) and β-tubulin (red).
TABLE 1.
NONTYPEABLE HAEMOPHILUS INFLUENZAE BINDING TO AND TRANSMIGRATION ACROSS WELL-DIFFERENTIATED PRIMARY AIRWAY EPITHELIAL CELLS ARE INCREASED BY RHINOVIRUS INFECTION
| Infection | Bacteria Associated with Cells at 24 Hours Postinfection (cfu/well) | Bacteria Recovered from Basolateral Chamber (cfu/well) | |
|---|---|---|---|
| 12 h | 24 h | ||
| Medium control | 450 ± 100 | 0 | 0 |
| Sham | 360 ± 25 | 0 | 0 |
| RV39 | 32000 ± 2600 | 400 ± 83 | 7600 ± 564 |
| RV1B | 24000 ± 4528 | 280 ± 52 | 4200 ± 672 |
Definition of abbreviations: NTHi = Nontypeable Haemophilus influenzae; RV = rhinovirus.
Data represent mean ± SEM. Six independent experiments were performed in duplicate. Differences between medium- or sham-treated and RV-infected cells were statistically significant (P < 0.05, one-way analysis of variance).
To facilitate mechanistic studies, we also tested whether RV promotes transmigration of bacteria across polarized 16HBE14o- human airway epithelial cells. These cells are readily available and form tight junctions similar to primary epithelial cells when grown on a semipermeable membrane (25). 16HBE14o- cells grown on semipermeable membranes were infected apically with RV39 (MOI of 1 for 1 h) and incubated for an additional 24 hours. NTHi were added to the apical chamber and the basolateral media were sampled at different time intervals to detect bacteria. Uninfected cultures and sham-infected cultures were used as negative controls. Cells treated with occludin peptide for 30 minutes were used as a positive control. Occludin peptide treatment induced a 44–48% reduction in RT compared with medium-treated controls, consistent with earlier studies (28, 29). For negative controls (cells treated with either medium or sham), bacteria were not recovered from the basolateral media until 12 hours after adding the bacteria (Table 2). Cultures treated with occludin peptide for 30 minutes, or infected with RV for 24 hours, showed bacteria in the basolateral chamber within 1 hour of incubation. Basolateral chamber bacterial load increased with the time of incubation. Bacteria were not recovered from the basolateral chamber of cells infected with UV-irradiated RV39, indicating that replicating virus is required for increasing bacterial transmigration. These results indicate that infection with replicative RV facilitates transmigration of bacteria across polarized 16HBE14o- cells, as in primary cells. To determine whether the rate of migration depends on bacterial species, we then examined the effect of RV infection on the transmigration of other strains of NTHi and other bacterial species, such as P. aeruginosa and S. aureus. NTHi isolates 5P19H1, and 45P9H1 and S. aureus each showed a similar rate of transmigration, comparable to that of NTHi 6P5H (Table 3). P. aeruginosa, which can cause cell damage, transmigrated across both sham- and RV-infected cells, though at a higher rate in RV-infected cells. Together, these observations suggest that RV-facilitated transmigration of bacteria across polarized cell monolayers is not specific to one species of bacteria.
TABLE 2.
RHINOVIRUS FACILITATES MIGRATION OF NONTYPEABLE HAEMOPHILUS INFLUENZAE FROM THE APICAL TO THE BASOLATERAL SURFACE OF POLARIZED 16HBE14O- CELLS
| Infection | Bacteria Recovered from Basolateral Chamber (log cfu/well) | |||||
|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 2 h | 3 h | 6 h | 12 h | |
| Medium | 0 | 0 | 0 | 0 | 0 | 1.34 ± 1.65 |
| Sham | 0 | 0 | 0 | 0 | 0 | 2.25 ± 1.84 |
| RV39 | 0.6 ± 1.2 | 0.8 + 1.6 | 1.89 ± 2.37 | 2.93 ± 2.48 | 5.15 ± 0.96 | 9.30 ± 0.51 |
| UV-RV39 | 0 | 0 | 0 | 0 | 0.43 ± 1.25 | 2.67 ± 2.31 |
| Occludin peptide | 0 | 1.37 ± 1.71 | 2.91 ± 2.44 | 4.23 ± 2.47 | 5.86 ± 1.25 | 8.95 ± 0.46 |
Definition of abbreviations: RV = rhinovirus; UV = ultraviolet.
Data represent mean ± SEM. Experiments were repeated three times in six replicates.
TABLE 3.
RHINOVIRUS FACILITATES MIGRATION OF BACTERIA FROM THE APICAL TO THE BASOLATERAL SURFACE OF POLARIZED 16HBE14O- CELLS
| Bacteria Recovered from Basolateral Chamber (log cfu/ml)
|
||||||
|---|---|---|---|---|---|---|
| Bacteria | Sham | RV39 | ||||
| 1 h | 3 h | 6 h | 1 h | 3 h | 6 h | |
| NTHi 5P19H1 | 0 | 0 | 0 | 1.56 ± 1.28 | 2.54 ± 2.08 | 5.28 ± 0.66 |
| NTHi 45P9H1 | 0 | 0 | 0 | 0.92 ± 1.12 | 2.78 ± 1.53 | 5.49 ± 0.94 |
| P. aeruginosa | 0 | 0.98 + 1.20 | 3.78 + 0.39 | 3.89 ± 0.59 | 5.15 ± 0.74 | 7.11 ± 0.89 |
| S. aureus | 0 | 0 | 0 | 1.06 ± 1.31 | 2.74 ± 1.43 | 5.35 ± 0.75 |
Definition of abbreviations: NTHi = Nontypeable Haemophilus influenzae; P. aeruginosa = Pseudomonas aeruginosa; RV = rhinovirus; S. aureus = Staphylococcus aureus.
Data represent mean ± SEM. Experiments were repeated three times in triplicate.
Rhinovirus Reduces RT
RV could facilitate transmigration of bacteria either by causing cell death or by disrupting tight junctions. To examine the capacity of RV to disrupt tight junctions, we measured RT in both primary human mucociliary differentiated airway epithelial cells and 16HBE14o- cultures after RV infection. Primary airway epithelial cells differentiated into a mucociliary phenotype, with an RT of 600–800 Ω · cm2, were infected apically with RV (MOI of 1) or an equal of volume of sham. After 5 hours, the infection medium was replaced with fresh medium and incubated further for 16 hours. The basolateral media were collected for detection of lactose dehydrogenase (LDH) activity (an indicator of cytotoxicity), and the RT of cell cultures measured using a voltmeter. Cells treated with medium alone served as control. Media-treated cells showed minimal LDH activity. Cell lysate, which was used as a positive control for LDH activity, showed sevenfold greater activity than the medium control. Both sham- and RV-infected cells showed LDH levels similar to medium-treated controls, indicating that RV did not induce cytotoxic effects in well-differentiated cells (Figure 2A). On the contrary, epithelial cells infected with all three serotypes of RV showed moderate but statistically significant decreases in RT compared with sham-treated controls (Figure 2B). Replication-deficient UV-treated virus had no effect on RT, indicating that viral replication is required for the reduction in RT (Figure 2C). We ultimately repeated the experiments with cells obtained from eight donors and in all cases we observed a similar reduction in RT in response to RV39 infection (data not shown).
Figure 2.
Rhinovirus (RV) decreases the transepithelial resistance (RT) of well-differentiated primary airway epithelial cells. Primary cultures grown at air/liquid interface were infected apically with (A) RV or (C) ultraviolet-treated RV at one multiplicity of infection and incubated for 5 hours. Infection medium was then removed, and the cells further incubated for 24 hours. Lactate dehydrogenase activity in the basolateral medium (A) and the cell culture RT (B and C) were measured. Cell lysate used in (A) was generated from representative media-treated cells. Results represent the mean of three independent experiments performed in triplicate; bars represent SEM. Statistical differences between experimental groups were determined by one-way analysis of variance (*P < 0.05).
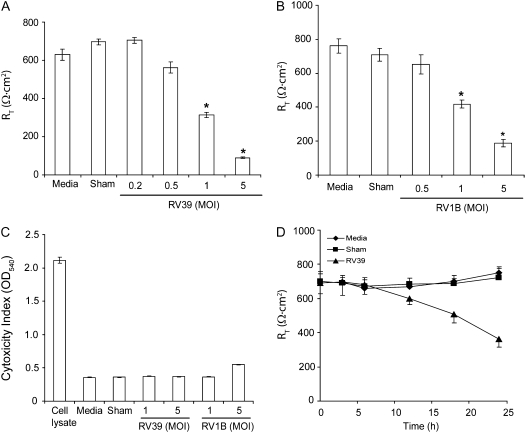
To confirm the results obtained with primary cultures, and to examine the feasibility of using polarized 16HBE14o- cells in subsequent experiments, we infected monolayers of 16HBE14o- cells with RT of 800–1000 Ω · cm2 with RV39 or RV1B at MOIs ranging from 0.2 to 5 for 1 hour. The infection medium was removed, incubation continued for another 24 hours, and RT was measured. RV39 and RV1B each decreased RT in a dose-dependent manner (Figures 3A and 3B). Effects on RT were greater in 16HBE14o- compared with primary cells. The 16HBE14o- cell line, a clonal isolate that likely represents one cell type, in contrast to heterogeneous primary cultures, does not produce mucus, which may interfere with viral infection. At the same time, none of the RV-infected cell cultures, except those infected with RV1B at 5 MOI, showed detectable amounts of LDH in the basolateral medium (Figure 3C). Based on these results, we chose to use RV at an MOI of 1.0 for subsequent experiments, as this concentration reduced RT without inducing cytotoxic effects.
Figure 3.
RV decreases the RT of polarized 16HBE14o- cells in a dose- and time-dependent manner. Cells grown in transwells were infected apically with (A) RV39 or (B) RV1B at doses ranging from 0.25 to 5 MOI and incubated for 1 hour. Infection medium was removed, incubation continued for another 23 hours and RT was measured. Cytotoxic effect was determined by measuring LDH levels in basolateral media (C). The time course of RV-induced reductions in RT was determined by infecting polarized cell monolayers with RV39 at MOI of 1 for 1 hour, replacing the infection media with fresh cell culture media, and measuring RT measured at the indicated times (D). Data represent the mean and standard error of four independent experiments carried out in triplicate (P < 0.05, analysis of variance).
To determine the kinetics of RV-induced reductions in RT, polarized 16HBE14o- cells were infected with RV39 (MOI of 1 for 1 h) and RT monitored at different time intervals up to 24 hours. A reduction in RT was not observed until 12 hours (Figure 3D). Next, we infected polarized cells with UV-irradiated RV39 (1 or 5 MOI) or equivalent volumes of sham. UV-irradiated RV did not affect the RT of polarized cells even at 5 MOI (data not shown). Taken together with results from primary cells, these data suggest that replication rather than initial binding or endocytosis of RV is required to cause significant reductions in RT.
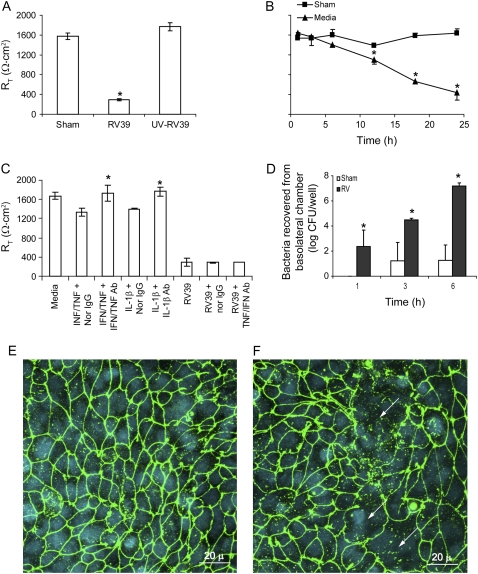
RV-induced Reductions in RT Are Not Mediated by Proinflammatory Cytokines
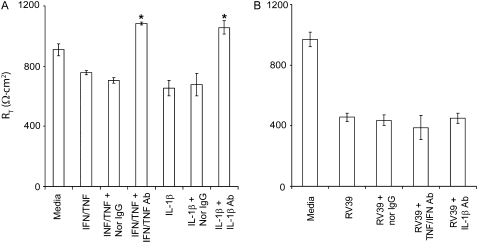
The proinflammatory cytokine IL-1β and a combination of TNF-α and IFN-γ have been shown to decrease the RT of differentiated airway epithelial cells (26). To examine whether cytokines released in response to RV infection mediate the observed reduction in RT, we infected 16HBE14o- airway epithelial cells with RV in the presence or absence of neutralizing antibodies to IL-1β, TNF-α, and IFN-γ alone or in combination. Normal IgG was used as a negative control, and recombinant IL-1β, TNF-α, and IFN-γ were used as positive controls. Polarized 16HBE14o- cells incubated with IL-1β or a combination of TNF-α and IFN-γ demonstrated a 20% reduction in RT. These reductions were completely reversed by their respective neutralizing antibodies (Figure 4A). Normal IgG did not affect RT. In contrast, none of the neutralizing antibodies tested reversed the effect of RV on RT (Figure 4B), suggesting that the cytokines released in response to RV infection do not mediate the observed reduction in RT.
Figure 4.
RV-induced reductions in RT are not mediated by proinflammatory cytokines. Polarized 16HBE14o- human bronchial epithelial cells were either treated with proinflammatory cytokines (A) or infected with RV for 24 hours (B) in the presence or absence of neutralizing antibodies to IFN-γ and TNF-α, neutralizing antibody to IL-1β, or normal IgG. RT was measured as described above. Results represent the mean of three independent experiments carried out in triplicate; bars represent SEM (P < 0.05, analysis of variance).
Apoptosis Does Not Contribute to RV-induced Reductions in RT
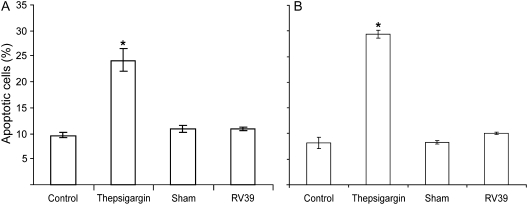
Though RV infection is notable for the lack of cytotoxicity relative to other respiratory viruses, such as RSV and influenza (14, 30, 31), RV has been shown to induce apoptosis by activating caspase 3 and caspase 9 and poly(ADP-ribose) polymerase activation in nonpolarized airway epithelial cells (32). To examine whether RV causes apoptosis in primary airway epithelial cells differentiated into mucociliary phenotype and in polarized 16HBE14o- cells, we infected these cultures with RV39 (MOI of 1) or sham, as described above, and determined the population of apoptotic cells by staining with annexin V and propidium iodide followed by flow cytometric analysis. As a positive control we treated cells with thepsigargin for 16 hours. For both primary airway epithelial cells and polarized 16HBE14o- cells, control, sham-infected and RV-infected cells showed approximately 10% apoptotic cells, and there was no significant increase in the apoptotic cell population following RV infection (Figures 5A and 5B). On the other hand, thaspsigargin-treated cell cultures showed 24.1 and 29.3% apoptotic cells in primary and 16HBE14o- cells, respectively. These results suggest that RV does not cause significant apoptosis in differentiated or polarized airway epithelial cells, and hence, the observed reductions in RT in RV-infected cells cannot be attributed to apoptosis.
Figure 5.
Apoptosis does not contribute to rhinovirus (RV)-induced reductions in transepithelial resistance (RT). (A) Primary diffentiated airway epithelial cells or (B) polarized 16HBE14o- human bronchial epithelial cells were infected with RV39 or sham or treated with 5 μM thepsigargin for 1 hour. Media-treated cells were used as negative controls. Cells were stained with mixture of FITC-annexin V and propidium iodide and analyzed by flow cytometry. Results represent the mean of three independent experiments performed in triplicate; bars represent mean ± SEM (P < 0.05, analysis of variance).
RV Increases Paracellular Permeability of Polarized Epithelial Cells
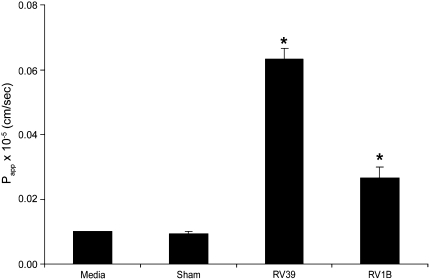
To correlate RV-induced reductions in RT with the barrier function of tight junctions, inulin permeability was measured after RV39, RV1B, or sham infection in polarized 16HBE14o- cells. Uninfected cultures were used as negative controls. Both RV39 and RV1B caused significant increases in permeability, as expressed by the permeability coefficient Papp, compared with uninfected or sham-infected cultures (Figure 6). These results indicate that RV-induced reductions in RT are associated with a functional increase in permeability to solute. This may occur as a result of disruption in the function of tight junctions.
Figure 6.
Effect of RV on the paracellular permeability of polarized epithelial cells to inulin. Polarized 16HBE14o- cells were infected with RV39 or RV1B at MOI of 1 for 24 hours. FITC labeled-inulin was added to the apical chamber, the basolateral chamber was sampled at different time intervals for fluorescence, and Papp was calculated. Results represent the mean of three independent experiments carried out in quadruplicate; bars represent SEM (P < 0.05, analysis of variance).
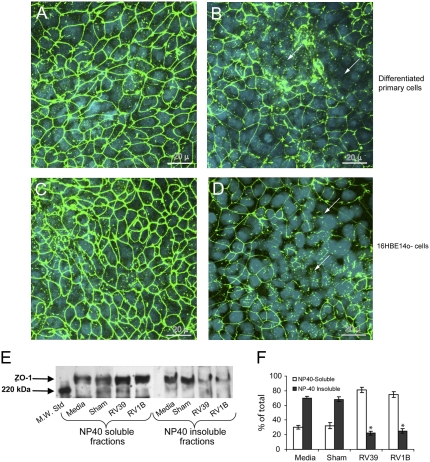
RV Causes Redistribution of ZO-1
Based on the effects of RV on RT and Papp, we hypothesized that RV redistributes or degrades tight junction proteins. Well-differentiated primary human airway epithelial cell cultures and 16HBE14o- cells were infected with RV39 or sham and immunostained with antibody to ZO-1, a protein of the tight junction complex, or normal IgG. In sham-infected cell cultures, ZO-1 was observed on the cell periphery (Figures 7A and 7C). In contrast, cultures infected with RV for 24 hours showed patches of cells with diffuse ZO-1 staining in the cytoplasm but no ZO-1 around the periphery, indicating dissociation of ZO-1 from the tight junction complex (Figures 7B and 7D). Similar results were observed when cells were infected with RV1B instead of RV39 (not shown). Cell cultures incubated with normal IgG instead of ZO-1 did not show signal, indicating specificity of the ZO-1 antibody.
Figure 7.
Rhinovirus (RV) infection reduces transepithelial resistance by dissociating zona occludins (ZO)-1 from tight junction complex. Well-differentiated (A and B) primary or (C and D) polarized 16HBE14o- human bronchial epithelial cells were infected with either (A and C) sham or (B and D) RV39 and incubated for 24 hours as described above. Cells were fixed in cold methanol and immunostained with antibody to ZO-1 (green). Nuclei were stained with DAPI (blue) (Sigma-Aldrich, St. Louis, MO). Arrows represent dissociation of ZO-1 from the tight junction complex. NP40 soluble and insoluble fractions from medium-treated, sham, RV39, or RV1B-infected polarized 16HBE14o- cells were subjected to Western blot analysis with (E) antibody to ZO-1. (F) A representative blot from three independent experiments is presented. Group mean data from three independent experiments (bars represent mean ± SEM; P < 0.05, analysis of variance).
To confirm the dissociation of ZO-1 from the tight junction complex, NP-40 soluble (cytoplasm) and NP-40 insoluble (cytoskeleton) fractions were prepared from 16HBE14o- cultures infected with RV39 or RV1B, and proteins were subjected to Western blot analysis with antibody to ZO-1. The ZO-1 bands were quantitated by densitometry. Uninfected and sham-infected cultures showed intense ZO-1 bands in the NP-40 insoluble fraction, corresponding to approximately 70% of the total (Figure 7E, and 7F). In contrast, RV-infected cultures showed light bands corresponding to approximately 20% of the total ZO-1 in the NP-40 insoluble fraction and relatively intense bands in the NP-40 soluble fraction. These results confirmed the dissociation of ZO-1 from cytoskeletal tight junction complexes to the cell cytoplasm following RV infection.
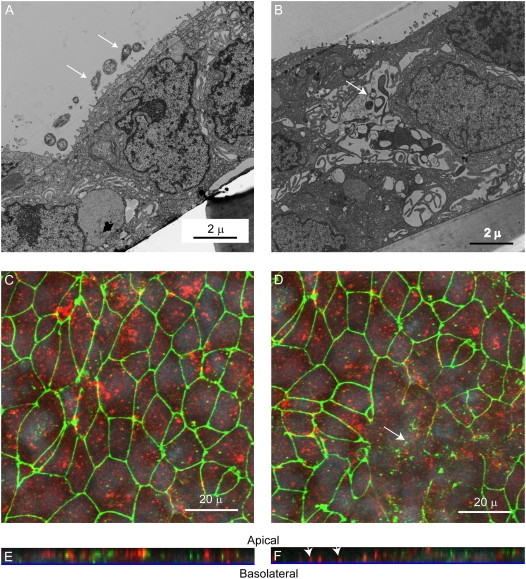
RV Causes Transmigration of Bacteria by the Paracellular Route
As noted above, our infection assays indicated that RV increased transmigration across well-differentiated airway epithelial cells. Because RV infection increased inulin permeability and altered tight junction complexes, we reasoned that bacteria transmigrate in RV-infected cultures by the paracellular route. To examine this, transmission electron microscopy was performed on 16HBE14o- cells infected with RV39 or sham for 24 hours, followed by incubation with NTHi 6P5H for 3 hours. Sham-infected cells appeared to have intact tight junctions, with bacteria mainly on the apical surface (Figure 8A). In contrast, RV-infected cells showed bacteria between the cells where tight junctions appeared to be disrupted (Figure 8B). To confirm these results, confocal fluorescence microscopy was conducted on cultures immunostained with antibodies against both ZO-1 and NTHi. Apical views of sham-infected cultures showed a normal distribution of ZO-1, and bacteria was found mainly on the apical surface (Figure 8C). Z-sections, created from serial optical sections taken through the whole thickness of the culture, also showed bacteria on the apical surface (Figure 8E). As noted previously, RV-infected cells showed altered ZO-1 expression (Figure 8D). Further, the Z sections showed bacteria located at the culture basolateral surface in an area devoid of ZO-1 staining (Figure 8F). These results indicate that RV infection facilitates transmigration of bacteria by the paracellular route.
Figure 8.
Rhinovirus (RV) promotes transmigration of bacteria by the paracellular route. 16HBE14o- human bronchial epithelial cells grown as polarized monolayers were infected apically with (A) sham or (B) RV39 for 24 hours. NTHi (0.1 ml of 1 × 109 cfu/ml) was added to the apical surface and incubated for 3 hours. Cells were fixed and processed for transmission electron microscopy. Arrows point to bacteria. Cultures were immunostained with antibodies to zona occludins (ZO)1 (green) and nontypeable Haemophilus influenzae (NTHi) (red) and analyzed by confocal microscopy, taking sections every 0.5 μm. (C and D) Apical view of sham- and RV- infected cells, respectively. (E and F) Z-sections of sham- and RV- infected cells. Arrows in D indicate areas devoid of ZO-1. Arrows in F indicate bacteria at the basolateral surface.
RV Decreases RT in Calu-3 Cells
To test the effects of RV in another respiratory epithelial cell line, we cultured Calu-3 lung adenocarcinoma cells in transwells as polarized cell monolayers. Calu-3 cells with RT of 1500–1900 Ω · cm2 were apically infected with RV39 or UV-irradiated RV39 at MOI of 1 or equal volume of sham for 16 to 18 hours, as described for 16HBE14o- cells, and measured RT at the end of the incubation period. RV-infected cells showed a reduction in RT, but not cells infected with UV-irradiated RV or sham (Figure 9A). RV did not cause a significant reduction in RT until 12 hours after infection, consistent with the notion that viral replication is necessary for this effect (Figure 9B). Neutralizing antibodies to neither IL-1β nor a combination of TNF-α and IFN-γ restored RV-induced reductions in RT, indicating that the effect is not mediated by the release of these proinflammatory cytokines in response to RV infection (Figure 9C). Increased transmigration of NTHi isolate 6P5H was observed in RV-infected cultures relative to sham-infected cells (Figure 9D). Confocal microscopy revealed that, whereas sham-infected cultures showed the presence of ZO-1 around the periphery of most cells, RV-infected cells showed a loss of ZO-1 in scattered patches, although nuclei were visible (Figure 9E and 9F). Taken together, these results indicate that, as in bronchial epithelial cells, RV infection disrupts the barrier function of Calu-3 cells by dissociating ZO-1 from the tight junction complex.
Figure 9.
Rhinovirus (RV) disrupts the barrier function of Calu-3 cells. (A) Polarized monolayers of Calu-3 cells were infected with RV39, ultraviolet-irradiated RV39, or sham for 24 hours and transepithelial resistance (RT) was measured. (B) The time course of reductions in RT in response to RV infection. (C) In some experiments, monolayers were either treated with proinflammatory cytokines or infected with RV for 24 hours in the presence or absence of neutralizing antibodies to IFN-γ and tumor necrosis factor-α, neutralizing antibody to IL-1β or normal IgG. Calu-3 monolayers infected with either sham or RV39 for 24 hours were incubated apically with nontypeable Haemophilus influenzae isolate 6P5H for 6 hours. (D) Medium from the basolateral chamber was sampled for bacteria at 1, 3, or 6 hours. Monolayers infected with (E) sham and (F) RV39 for 24 hours were immunostained with antibody to zona occludins (ZO)-1 (green) and counterstained with DAPI (blue). Results represent the mean of three independent experiments performed in triplicate; bars represent mean ± SEM (P < 0.05, analysis of variance). Arrows in E and F point to the dissociation of ZO-1 from the periphery of cells.
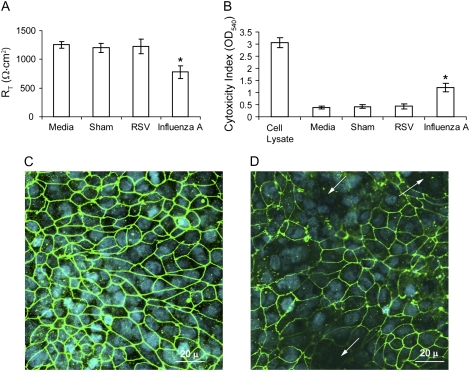
Effects of RSV and Influenza A Virus on Tight Junction Function
To examine whether other respiratory viruses disrupt the function of tight junctions similar to RV, we infected 16HBE14o- cells apically with RSV or influenza virus for 24 hours and determined RT, LDH activity, and ZO-1 distribution. RSV had no effect on either RT or LDH activity (Figure 10A and 10B). Corroborating the RT measurements, RSV-infected cells showed a distribution of ZO-1 similar to sham-infected cultures, indicating that RSV did not dissociate ZO-1 from tight junction complex (Figure 10C), In contrast, influenza A virus decreased RT by 39% and increased LDH activity nearly threefold compared with sham-infected cells. Confocal microscopy revealed not only the loss of ZO-1 in affected areas, but also the absence of cell nuclei (Figure 10D). These results indicate that influenza A virus reduces RT by inducing cell death rather than disrupting tight junctions. We then measured the transmigration of S. aureus in sham-, RSV- or influenza A-infected cell monolayers. Similar to sham-infected cells, no bacteria were recovered from the basolateral chamber of RSV-infected cell cultures up to 6 hours after bacterial inoculation. In contrast, 3.5 × 105 ± 1.2 × 105 cfu/ml were recovered 30 minutes after adding bacteria to influenza A-infected cultures. Such a high rate of bacterial migration from apical to basolateral surface occurs following exposure of the bare membrane after the loss of cells.
Figure 10.
Influenza A, but not RSV, causes a cytopathic effect in polarized 16HBE14o- human bronchial epithelial cells. Cells grown in Transwells were infected apically with RSV or influenza A virus and incubated for 1 hour Infection medium was removed, cells were further incubated for 23 hours and RT of cell cultures (A) and LDH levels in basolateral medium (B) were measured. In some experiments, cells were infected with RSV (C) or influenza A virus (D) as described above, fixed in methanol, and immunostained with antibody to ZO-1. Results represent the mean of three independent experiments performed in triplicate; bars represent mean ± SEM (P < 0.05, analysis of variance). Arrows in panel D show loss of both ZO-1 and nuclei.
RV Disrupts ZO-1 Distribution In vivo
We developed a mouse model of RV exposure using RV1B, a minor serotype RV. Intranasal inoculation of C57BL/6 mice with RV1B causes airways inflammation and hyperresponsiveness (27). We inoculated mice with RV1B or sham by the intranasal route (1 × 109 TCID50/ml) and lungs were harvested 24 hours later. Paraffin sections were immunostained with ZO-1 antibody and analyzed by confocal microscopy. In sham-inoculated animals, there was uniform distribution of ZO-1 in both large and small airways (Figures 11A and 11C). In contrast, RV1B-infected mice showed discontinuity in the distribution of ZO-1 (Figures 11B and 11D). Taken together, these results suggest that RV disrupts airway epithelial cell barrier function by dissociating ZO-1 from tight junctions.
Figure 11.
Rhinovirus (RV)1B disrupts airway epithelial tight junctions in vivo. C57BL/6 mice were infected with RV1B (5 × 107 TCID50) or an equal volume of sham. Mice were killed at 24 hours post-infection, and paraffin sections prepared from the lungs were deparaffinized, rehydrated, and immunostained with antibody to ZO-1. The bound antibody was detected by anti-rabbit IgG conjugated with AlexaFluor 488. (A and C) Large and small airways, respectively, from sham-infected mice. (B and D) Large and small airways, respectively, from RV1B-infected mice. Arrows represent absence of dissociation of zona occludins-1 from the tight junction complex.
DISCUSSION
Our results suggest that RV, which causes a majority of common colds, facilitates the transmigration of bacteria across the airway epithelium. Increased transmigration was accompanied by reduced RT and increased permeability to inulin. RV infection did not cause cytotoxicity or apoptosis. Exposure to sham HeLa cell supernatants or UV-irradiated RV did not compromise barrier function, indicating that the observed effect is a specific effect of replicating virus, and not a contaminant in the virus stock. Finally, we demonstrated that RV induces dissociation of ZO-1 from the tight junction complex in vitro and in vivo. In contrast, influenza A virus increased permeability of polarized airway epithelial cells by inducing cytotoxic effects, whereas RSV had no effect on either RT or ZO-1 distribution. These studies provide insight into a new mechanism by which RV may predispose the host to secondary bacterial infection, as occurs in otitis media (1, 12) and exacerbations of COPD (4–7).
RV has a limited capacity to cause lower airway infection, and stimulates a relatively mild inflammatory response in the upper respiratory tract. Unlike influenza virus and RSV, RV infection does not significantly damage the airway epithelium (14). Nevertheless, there is limited evidence suggesting that RV infection can increase binding of bacteria to and promote internalization of bacteria by epithelial cells. For example, RV infection increases the binding of S. pneumonia to bronchial epithelial cells by up-regulating expression of the platelet-activating factor receptor (19). RV infection promotes internalization of S. aureus by A549 pulmonary epithelial cells, which are otherwise nonpermissive (20). RV increases the expression of ICAM-1 (20, 33), which serves as a receptor for NTHi (34) as well as RV. Thus, it is conceivable that RV infection increases the adherence of NTHi to airway epithelial cells by increasing surface ICAM-1 expression. However, the effect of RV infection on the transmigration of NTHi across well-differentiated airway epithelial cells has not been observed previously.
Migration of bacteria from apical to basolateral surface in polarized epithelium can occur either by a transcellular or paracellular pathway. Polarized epithelial cells are not permeable unless they are damaged or their barrier function is disrupted. Hence, we examined the effect of RV on both RT and inulin permeability, as measured by Papp. RT, which correlates with the number of tight junction strands, does not necessarily correlate with paracellular permeability to noncharged solutes (26). On the other hand, increases in Papp represent a functional change in paracellular permeability and are always accompanied by reductions in RT. We found that RV significantly decreased RT and increased permeability to inulin 24 hours after infection. The observed reduction in RT did not occur until 8 hours after infection. Moreover, replication-deficient, UV-treated RV had no effect on RT. RV-induced changes in RT were most evident when infected cells were incubated at 33°C, the optimal temperature for RV replication (data not shown). It should be noted that, though 33°C is less than core body temperature, lower temperatures are commonly found in the proximal airways, especially when breathing cold air (as in winter) or at high levels of minute ventilation (as during exercise) (35). Finally, we showed that RV infection in mice leads to loss of ZO-1 in the proximal airway epithelium. Taken together, these data suggest that RV-induced reductions in RT are dependent on viral replication and are biologically relevant.
Recently, transient binding of particular viruses to the apical cell surface has been shown to disrupt tight junctions, leading to an increase in epithelial permeability, which in turn allows migration of virus to the basolateral surface where the major viral receptors are located. Initial binding of coxsackievirus, a member of the Picarnoviridae family, to decay-accelerating factor on the apical cell surface activates Abl kinase and triggers Rac-dependent actin rearrangement, thereby permitting virus migration across the tight junctions and interaction with its major receptor, coxsackievirus-adenovirus receptor (36–38). In contrast to our findings with RV, epithelial RT decreased within 15 minutes of coxsackievirus infection and returned to normal by 1 hour, indicating that initial binding of virus is sufficient to cause the observed changes. In addition, the VP8 subunit of rotavirus capsid protein VP4 transiently disrupts barrier function of the polarized intestinal epithelium, thus permitting the interaction of rotovirus with its basolateral integrin receptors, which is required for its entry into cells (38).
Based on RV-induced changes in RT and Papp, we examined the redistribution of ZO-1, a tight junction cytoplasmic adaptor protein that acts as a bridge between integral tight junction membrane proteins such as occludins, junctional adherence molecule and cytoskeletal proteins, and is present in the periphery of polarized epithelial cells. Immunofluorescence staining and immunoblots from RV-infected airway epithelial cells showed translocation of ZO-1 from the membrane to the cytoplasm, indicating dissociation of ZO-1 from the tight junction complex. Further, fluorescent confocal microscopy of airways from RV1B-inoculated mice showed discontinuity in the distribution of ZO-1. Taken together, these data suggest that RV disrupts airway epithelial cell barrier function by dissociating ZO-1 from tight junctions. Viral infection of epithelial cells has been shown to induce cytotoxicity, apoptosis, and production of pro-inflammatory cytokines, each of which has been shown to induce structural changes in tight junctions (26, 39, 40). During apoptosis in polarized epithelial cells, cysteine protease family caspases are activated, leading to proteolytic cleavage of the tight junction proteins occludin, ZO-1 and ZO-2. However, under the experimental conditions used, there was no evidence of cytotoxicity, as assessed by trypan blue exclusion assay (see Figure E1 in the online supplement) and LDH release, except at a high viral doses (5 MOI) 24 hours after incubation. Neutralizing antibodies to proinflammatory cytokines IL-1β, IFN-γ, and TNF-α had no effect on RV-induced disruption in barrier function. RV infection did not increase apoptosis, as assessed by annexin V staining. These results suggest that cytotoxic effects, pro-inflammatory cytokines, and apoptosis are not responsible for the observed tight junction disruption.
Intranasal challenge in human volunteers with rhinovirus has been shown to attenuate mucociliary clearance (41). Thus, it is conceivable that, in addition to increasing paracellular permeability, RV facilitates secondary bacterial infection of the airway epithelium by attenuating mucociliary clearance. Although this cannot easily be tested in an in vitro model, we developed an animal model of RV infection in which mice show persistence of viral RNA up to 4 days, mild neutrophilic lung inflammation, lung interferon production, and airways cholinergic hyperresponsiveness (27).
In summary, we have shown that RV disrupts the barrier function of airway epithelium and induces dissociation of ZO-1 from the cell membrane both in vitro and in vivo. The effect on barrier function appears to depend on viral replication. This study provides insight into a mechanism by which RV can predispose the host to secondary bacterial infection.
Supplementary Material
This work was supported by the National Institutes of Health grant HL82550 (M.B.H.)
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200801-136OC on September 11, 2008
Conflict of Interest Statement: U.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Q.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.C.G. received royalties for licensing cells used in this study through the University of California, San Francisco. M.B.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bulut Y, Guven M, Otlu B, Yenisehirli G, Aladag I, Eyibilen A, Dogru S. Acute otitis media and respiratory viruses. Eur J Pediatr 2007;166:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer-Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J 2008;27:589–594. [DOI] [PubMed] [Google Scholar]
- 3.Yano H, Okitsu N, Hori T, Watanabe O, Kisu T, Hatagishi E, Suzuki A, Okamoto M, Ohmi A, Suetake M, et al. Detection of respiratory viruses in nasopharyngeal secretions and middle ear fluid from children with acute otitis media. Acta Otolaryngol 2008;June 13:1–6. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:167–173. [DOI] [PubMed] [Google Scholar]
- 5.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. [DOI] [PubMed] [Google Scholar]
- 6.Bandi V, Jakubowycz M, Kinyon C, Mason EO, Atmar RL, Greenberg SB, Murphy TF. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol Med Microbiol 2003;37:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of copd. Chest 2006;129:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong D, Grimwood K, Carlin JB, Carzino R, Hull J, Olinsky A, Phelan PD. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol 1998;26:371–379. [DOI] [PubMed] [Google Scholar]
- 9.Collinson J, Nicholson KG, Cancio E, Ashman J, Ireland DC, Hammersley V, Kent J, O'Callaghan C. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 1996;51:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen HK, Hoiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax 1992;47:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen NT, Hoiby N, Mordhorst CH, Lind K, Flensborg EW, Bruun B. Respiratory infections in cystic fibrosis patients caused by virus, Chlamydia and Mycoplasma: Possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand 1981;70:623–628. [DOI] [PubMed] [Google Scholar]
- 12.Jacoby DB, Tamaoki J, Borson DB, Nadel JA. Influenza infection causes airway hyperresponsiveness by decreasing enkephalinase. J Appl Physiol 1988;64:2653–2658. [DOI] [PubMed] [Google Scholar]
- 13.Walsh JJ, Dietlein LF, Low FN, Burch GE, Mogabgab WJ. Bronchotracheal response in human influenza type A, Asian strain, as studied by light and electron microscopic examination of bronchoscopic biopsies. Arch Intern Med 1961;108:376–388. [DOI] [PubMed] [Google Scholar]
- 14.Tristram DA, Hicks W Jr, Hard R. Respiratory syncytial virus and human bronchial epithelium. Arch Otolaryngol Head Neck Surg 1998;124:777–783. [DOI] [PubMed] [Google Scholar]
- 15.Davison VE, Sanford BA. Adherence of Staphylococcus aureus to influenza A virus-infected madin-darby canine kidney cell cultures. Infect Immun 1981;32:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raza MW, Blackwell CC, Ogilvie MM, Saadi AT, Stewart J, Elton RA, Weir DM. Evidence for the role of glycoprotein G of respiratory syncytial virus in binding of Neisseria meningitidis to Hep-2 cells. FEMS Immunol Med Microbiol 1994;10:25–30. [DOI] [PubMed] [Google Scholar]
- 17.Sanford BA, Ramsay MA. Bacterial adherence to the upper respiratory tract of ferrets infected with influenza A virus. Proc Soc Exp Biol Med 1987;185:120–128. [DOI] [PubMed] [Google Scholar]
- 18.Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 2006;80:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizuka S, Yamaya M, Suzuki T, Takahashi H, Ida S, Sasaki T, Inoue D, Sekizawa K, Nishimura H, Sasaki H. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 2003;188:1928–1939. [DOI] [PubMed] [Google Scholar]
- 20.Passariello C, Schippa S, Conti C, Russo P, Poggiali F, Garaci E, Palamara AT. Rhinoviruses promote internalisation of staphylococcus aureus into non-fully permissive cultured pneumocytes. Microbes Infect 2006;8:758–766. [DOI] [PubMed] [Google Scholar]
- 21.Raza MW, El Ahmer OR, Ogilvie MM, Blackwell CC, Saadi AT, Elton RA, Weir DM. Infection with respiratory syncytial virus enhances expression of native receptors for non-pilate Neisseria meningitidis on Hep-2 cells. FEMS Immunol Med Microbiol 1999;23:115–124. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby P, Watson K, Bowman J, Taylor A, Riley TV, Smith DW, Lehmann D. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine 2007;25:2458–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sajjan US, Wang Q, Jia Y, LiPuma J, Hershenson MBA. Rhinovirus compromises tight junctions in differentiated airway epithelial cells and predisposes mice to secondary bacterial infection. Proc Am Thorac Soc 2007;4:A774. [Google Scholar]
- 24.Sajjan U, Keshavjee S, Forstner J. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infect Immun 2004;72:4188–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. Cftr expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 1994;10:38–47. [DOI] [PubMed] [Google Scholar]
- 26.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 2002;13:3218–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, et al. Human rhinovirus 1b exposure induces pi 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med 2008;177:1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everett RS, Vanhook MK, Barozzi N, Toth I, Johnson LG. Specific modulation of airway epithelial tight junctions by apical application of an occludin peptide. Mol Pharmacol 2006;69:492–500. [DOI] [PubMed] [Google Scholar]
- 29.Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P. A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol 2003;64:1530–1540. [DOI] [PubMed] [Google Scholar]
- 30.Giebink GS, Ripley ML, Wright PF. Eustachian tube histopathology during experimental influenza a virus infection in the chinchilla. Ann Otol Rhinol Laryngol 1987;96:199–206. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto S, Kawabata S, Nakagawa I, Okuno Y, Goto T, Sano K, Hamada S. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J Virol 2003;77:4104–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deszcz L, Gaudernak E, Kuechler E, Seipelt J. Apoptotic events induced by human rhinovirus infection. J Gen Virol 2005;86:1379–1389. [DOI] [PubMed] [Google Scholar]
- 33.Whiteman SC, Bianco A, Knight RA, Spiteri MA. Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J Biol Chem 2003;278:11954–11961. [DOI] [PubMed] [Google Scholar]
- 34.Avadhanula V, Rodriguez CA, Ulett GC, Bakaletz LO, Adderson EE. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect Immun 2006;74:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFadden ER Jr, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N, Solway J. Thermal mapping of the airways in humans. J Appl Physiol 1985;58:564–570. [DOI] [PubMed] [Google Scholar]
- 36.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie b viruses and adenoviruses 2 and 5. Science 1997;275:1320–1323. [DOI] [PubMed] [Google Scholar]
- 37.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 2006;124:119–131. [DOI] [PubMed] [Google Scholar]
- 38.Nava P, Lopez S, Arias CF, Islas S, Gonzalez-Mariscal L. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J Cell Sci 2004;117:5509–5519. [DOI] [PubMed] [Google Scholar]
- 39.Bojarski C, Weiske J, Schoneberg T, Schroder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci 2004;117:2097–2107. [DOI] [PubMed] [Google Scholar]
- 40.Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. Am J Physiol Cell Physiol 2004;286:C1213–C1228. [DOI] [PubMed] [Google Scholar]
- 41.Doyle WJ, Skoner DP, Fireman P, Seroky JT, Green I, Ruben F, Kardatzke DR, Gwaltney JM. Rhinovirus 39 infection in allergic and nonallergic subjects. J Allergy Clin Immunol 1992;89:968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.