Abstract
Imaging studies show that in normal language correlated activity between anterior and posterior brain regions increases as the linguistic and semantic content (i.e., from false fonts, letter strings, pseudo words, to words) of stimuli increase. In schizophrenia however, disrupted functional connectivity between frontal and posterior brain regions has been frequently reported and these disruptions may change the nature of language organization. We characterized basic linguistic operations in word and letter string processing in a region-of-interest network using structural equation modeling (SEM). Healthy volunteers and volunteers with schizophrenia performed an fMRI one-back matching task with real words and consonant letter strings. We hypothesized that left hemisphere network dysfunction in schizophrenia would be present during processes dealing with linguistic/semantic content. The modeling results suggest aberrant left hemisphere function in schizophrenia, even in tasks requiring minimal access to language. Alternative mechanisms included increases in right hemisphere involvement and increased top-down influence from frontal to posterior regions.
Keywords: Schizophrenia and language, Lateralization, Lexical-semantic processing, Imaging, Effective Connectivity, Modeling
1. Introduction
Studies of normal language organization using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) indicate that fronto-temporal regional interactions (i.e., correlated activity) in the language dominant left hemisphere are key in single word and letter string processing (e.g., Bokde, Tagamets, Friedman, & Horwitz, 2001; Price, Indefrey, & van Turennout, 1999; Ragland et al., 2004). Correlations between left anterior and posterior brain regions increase as stimuli increase in linguistic and semantic content: from false fonts to letter strings to pseudo words, and words (e.g., Bokde et al., 2001; Cohen et al., 2000; Horwitz & Braun, 2004; Mechelli et al., 2005; Price et al., 1999; published abstract, Tagamets, Chalmers, Horwitz, & Friedman, 2000a). These functional word-level relationships have not been characterized in schizophrenia. Because these regional interactions covary with normal language function, disruptions may be related to language aberrations observed in schizophrenia (reviews, Covington et al., 2005; DeLisi, 2001; Goldberg et al., 1998; Kuperberg & Caplan, 2003).
In normal language, the strongest left-sided functional connectivity between the frontal cortex and posterior language regions occurs for real words, even when the task does not require any linguistic or semantic processing. Semantic processing is thought to take place even when it is not required for performance (Bokde et al., 2001; Petersen & Fiez, 1993; Price, Winterburn, Giraud, Moore, & Noppeney, 2003; Tagamets, Novick, Chalmers, & Friedman, 2000b). For example, using a one-back matching task with real words (fMRI; Bokde et al., 2001), we found significant functional connectivity of both the left ventral inferior frontal gyrus (VIFG) and dorsal inferior frontal gyrus (DIFG) with posterior visual (BA 18 &19) and temporal regions (BA 20, 21, & 37). In contrast, consonant letter strings elicited only DIFG connectivity with the same posterior language regions. Because the VIFG is thought to mediate semantic processing (e.g., Buckner & Koutstaal, 1998; Demb et al., 1995), these results suggest that semantic processes were automatically recruited to support low-level operations with real words.
It is an open issue in the literature whether correlated anterior/posterior interactions in schizophrenia vary as a function of the linguistic content as observed for language tasks using healthy subjects. In schizophrenia, many reported functional disconnections are in the left hemisphere, and generally in tasks that involve language (verbal fluency, Boksman et al., 2005; encoding and recall, word lists, Fletcher, McKenna, Friston, Frith, & Dolan, 1999; lexical decision and retrieval, Foucher et al., 2005; sentence completion, Lawrie et al., 2002; word and letter category decisions, Jennings, McIntosh, Kapuv, Zipursky, & Houle, 1998; semantic judgments, Kim et al., 2005; word encoding, Ragland et al., 2004; word encoding, Wolf et al., 2007). Given these reports, we expected that left hemisphere language networks would be atypically organized. An additional consideration was that atypical lateralization could modify the patterns of anterior/posterior correlations in schizophrenia. This consideration was motivated by evidence for reduced lateralization in schizophrenia (review, Gur & Chin, 1999) and proposals that decreased functional lateralization may be due to abnormally high activity in the right hemisphere, rather than lower than normal activity on the left (Artiges et al., 2000; Jaynes, 1977; Sommer et al., 2001; Woodruff et al., 1997).
We hypothesized that in schizophrenia (1) left hemisphere dysfunction underlies the most basic processes dealing with linguistic/semantic content, and (2) that compensatory mechanisms include increased right hemisphere involvement and increased top-down influence from frontal into posterior regions. These hypotheses were evaluated by characterizing (a) the lateralization of processing, (b) the bottom-up versus top-down effects, and (c) the profiles of regional coupling and decoupling indexed by the computed effective connectivity for word and letter string stimuli. Whereas functional connectivity is used for characterizing regional interactions with the task-related correlations or covariance, effective connectivity extends the characterization to testing hypothesized causal models of regional coupling (Penny, Stephan, Mechelli, & Friston, 2004). We used structural equation modeling (SEM) to model fMRI task-related blood oxygen level dependent (BOLD) signal changes to reflect network interactions. Our goals were to quantify and qualify the word-level language network organization and evaluate the differences between persons with schizophrenia and healthy volunteers. We employed a one-back visual matching task in which the task demands elicited the relatively automatic aspects of linguistic and semantic access.
2. Materials and methods
2.1. Participants
Eight persons between the ages of 21 and 42 (4 males, 4 females [5 outpatients, 3 inpatients]), mean age = 32), with a DSM-IV diagnosis of schizophrenia were included in the study. Psychiatric symptoms as assessed with the Brief Psychiatric Rating Scale (BPRS) were moderate in severity (range = 23 to 31). Trained raters conducted the clinical interviews (reliability = 0.86). Volunteers with schizophrenia had 12.75 mean years of education (range = 12 to 15) and their mean years of paternal education was 14 (range = 9 to 19). Ten healthy volunteers between the ages of 21 and 44 (6 females, 4 males, mean age = 32) were included in the study. Their mean years of education was 14.90 (range = 12 to 19) and mean years of paternal education was 14.33 (range = 12 to 19). All participants were free of major medical conditions. Any participant with metal in the body was excluded because of safety in the MR magnet. All participants were right-handed native English speakers.
The volunteers with schizophrenia were clinically stable; all were taking an atypical antipsychotic and each was treated with their same antipsychotic and dose for the previous 2 months. They were not currently taking medication other than that for schizophrenia. Two research psychiatrists reached a consensus diagnosis of schizophrenia based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-1; First, Spitzer, Gibbon, & Williams, 2002), a general psychiatric interview, and information from 2–3 months of independent clinician observation.
After an initial phone screening, all HV were assessed with the SCID and the Structured Interview for DSM-IV Personality Disorders (SID-P,Pfhol, Blum, & Zimmerman, 1997). Healthy volunteers had no psychiatric illness and no first-degree relatives with psychiatric disorders, no current substance/alcohol abuse or dependence, and no diagnosable neurological condition. The Institutional Review Boards of the University of Maryland School of Medicine and the Johns Hopkins School of Medicine approved the study protocol. Prior to giving consent, schizophrenic volunteers completed an ‘Evaluation to Sign Consent’ questionnaire to probe their understanding of various aspects of the study. Only patients who were competent and judged clinically capable of understanding the risks involved in the study were selected from the Residential Research Program and the Schizophrenia Related Disorders Program of the Maryland Psychiatric Research Center in Baltimore, MD, USA. Family members or caregivers were involved in the information process when available. All volunteers were fully informed regarding the nature of the protocol, and afterwards each of them gave informed consent.
2.2. Behavioral Task
Participants performed a block-design one-back visual matching task. They viewed sequences of common four-character words (Words condition; e. g., TONE), or 4-character consonant letter strings (Letter Strings condition; e. g., QTVP), with separate runs for Words and Letter Strings. Control blocks had sequences of single geometric shapes (● or □). For all task conditions, participants were instructed to press a button if the current stimulus matched the one immediately previous. The stimuli were centrally presented at an ISI (Inter-stimulus Interval) of one per 1000 ms, 200 ms duration. There were 120 control trials and 90 task trials each for Words and Letter Strings. There were 30 trials in a block, and the series began and ended with a control block. The block design provided a continuous series of each stimulus for computing within-task correlations for contiguous volumes (details, 2.4.2.).
2.3. fMRI
2.3.1. fMRI acquisition
Volumes were acquired with a Siemens Vision 1.5 T scanner with a fast gradient system. Interleaved echo-planer images (EPI) of 20 axial 5 mm thick slices (1 mm gap) were obtained. Matrix size was 64 × 64 and in-plane resolution was 3.75 × 3.75 mm, with TR= 2000 ms (repetition time), TE = 40 ms (echo time), flip angle = 90°, and a 240 × 240 mm FOV. Scanning for each series started 12 seconds before data collection and task performance began in order to allow spin saturation to reach steady state, and 105 volumes were collected for each fMRI time series.
2.3.2. Preprocessing
Preprocessing using SPM2 (Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm2) was done as follows: a) differences in slice acquisition times were corrected, b) motion effects were corrected by coregistration to the first image in each series, c) volumes were normalized to a standard template (MNI, Friston et al., 1995) and d) volumes were smoothed by convolution with a Gaussian kernel function (FWHM; Full Width Half Maximum = 10 mm).
2.3.3. Subtraction analysis
Subtraction analyses were performed using the general linear model in SPM2. Between group comparisons were performed using a mask to include only voxels that are activated in the group being compared at a p < 0.001 level, uncorrected for multiple comparisons. For example, the HV –SZ comparisons were masked by regions in which the HV had activations at a level of p < 0.001 or better. This method yields regions that are activated in one group and not the other. Supra-threshold voxels from this analysis that survived at p < 0.05, corrected for multiple comparisons, and an extent threshold of at least 10 voxels, are reported in Table 1.
Table 1.
Between-Group Subtraction Comparisons Words and Letter Strings
| Words | ||||||
|---|---|---|---|---|---|---|
| x | y | z | k | p (corrected) | ||
| HV-SZ | IT/TEO | −44, | −72 | −16 | 264 | 0.010 |
| SZ-HV | No supra-threshold voxels | |||||
| Letter Strings
| ||||||
| HV-SZ | V4 | 24 | −80 | −20 | 134 | 0.000 |
| V1/V2 | −20 | −96 | −12 | 127 | 0.006 | |
| SZ-HV | No supra-threshold voxels | |||||
2.4. Structural Equation Modeling
2.4.1. Regions of interest
Six model ROIs for each hemisphere were chosen based on the task demands of one-back matching: VI/V2 (Early Visual Cortex), V4 (Extrastriate Association Cortex), IT (Posterior Inferior Temporal Cortex), VIFG (Ventral Inferior Frontal Gyrus; BA 47), DIFG (Dorsal Inferior Frontal Gyrus; BA 44/45), and HC (Hippocampal Complex). The area for sensory input into the visual cortex, the LGN (Lateral Geniculate Nucleus), was modeled as a latent variable with the variance fixed at 1. Word and letter string processing involves basic visual processing (Bokde et al., 2001; Price et al., 1999), automatic orthographic, phonologic, and semantic processing at the word level (Neely, 1991; Tagamets et al., 2000b). The primary (V1) and secondary (V2) visual areas, and the extrastriate association area (V4), respond selectively to visual features of stimuli (Ungerleider & Haxby, 1994). Word identification (Poldrack et al., 1999) and prelexical processing of word-like stimuli (Binder et al., 2003; Cohen et al., 2000; Fiez, 1997) are thought to occur in the left inferior temporal cortex. Whereas Broca’s area (BA 44/45) is historically associated with speech production networks, as are other portions of the middle frontal gyrus (BA 46, BA 9, BA 6; Brannen et al., 2001), evidence suggests more specific involvement of the midventral region (BA 45) and posterior ventrolateral region (BA 44) in phonological processing (Demonet et al., 1992; Paulesu, Frith, & Frackowiak, 1993; Zatorre, Meyer, Gjedde, & Evans, 1996). The left anterior inferior frontal cortex (BA 47) supports multiple semantically related processes: semantic priming (Buckner & Koutstaal, 1998; Demb et al., 1995), and the control of retrieval, evaluation, and manipulation of meaning (Bokde et al., 2001; Buckner & Koutstaal, 1998; Devlin et al., 2003; Dobbins et al., 2002; Petersen, Fox, Posner, Mintun, & Raichle, 1988). Evidence suggests the HC regions, the entorhinal cortex and the perirhinal cortex, integrate visual, lexical, semantic, and encoding and retrieval aspects of word processing (Halgren et al., 2006; Hoenig & Scheef, 2005).
2.4.2. Extracting regions of interest
Time series from 4 mm ROIs (i.e. about one FWHM) were extracted for each participant for six each left and right hemisphere model regions. The center coordinate of each radius was based on a local maximum from the main effects analysis across conditions and groups. Then the precise center for each condition and group was chosen as the maximum within a 10 mm radius of this location to allow for some variability in exact locations and avoid bias for any single group or condition. It should be noted that these coordinates (see Table 2) are within a few mm of those published in our study in healthy volunteers (Tagamets et al., 2000b). To account for differences in regional base activity levels, each ROI time series was normalized to a common mean over the entire run. A hemodynamic delay and effects of transitions between blocks were accounted for by shifting the beginning points of task blocks by 6 seconds and dropping the first two and the last data point from each block (Winder, Cortes, Reggia, & Tagamets, 2007). The SEMs were fit to ROI correlations computed from these within task time series.
Table 2.
Regions of Interest Montreal Neurological Institute Template Coordinates
| Words
| ||||
|---|---|---|---|---|
| Hemisphere | ||||
| Left
|
Right
|
|||
| Group | HV | SZ | HV | SZ |
| Area | ||||
| V1/V2 | −10,−100,−4 | −10,−100,−4 | 32,−98,4 | 32,−98,4 |
| V4 | −32,−88,−14 | −32,−88,−14 | 36,−92,−10 | 36,−92,−10 |
| IT | −48,−64,−16 | −48,−64,−16 | 46,−70,−16 | 46,−70,−16 |
| VIFG | −50,34,−16 | −48,34,−14 | 42,32,−20 | 48,34,14 |
| DIFG | −44,6,24 | −48,8,24 | 46,10,28 | 40,8,34 |
| HC | −22,−22,−12 | −22,−22,−12 | 16,−34,−4 | 20,−30,−2 |
|
Letter Strings
| ||||
| V1/V2 | −18, −90, −14 | −6,−100,−10 | 30,−90,−14 | 32,−98,6 |
| V4 | −30,−80,−6 | −30,−88,−14 | 44,−86,−10 | 34,−92,−10 |
| IT | −54,−60,−18 | −46,−54,−34 | 42,−78,−20 | 46,−70,−10 |
| VIFG | −48,40,−12 | −46,38,−12 | 44,40,−12 | 36,40,−18 |
| DIFG | −40,4,30 | −54,−2,22 | 42,2,24 | 48,2,28 |
| HC | −22,−20,−12 | −10,−20,8 | 16,−38,−12 | 20,30,6 |
2.4.3. Regional connections
The model paths represented the anatomical connectivity between regions. Our generic model comprised a simplified version of the ventral visual object or “what” pathway, whose structures and connections are supported by both nonhuman animal and human studies (nonhuman primates, Ungerleider & Mishkin, 1982; Ungerleider & Haxby, 1994; human, Catani, Jones, Bonato, & ffytche, 2003; Mori et al., 2002; Tanaka, 1997). The visual one-back matching task activates the primary visual cortex (V1) as it receives sensory information from the lateral geniculate nucleus, and in succession projections go to the V2, V4, the inferior temporal cortex, and the lateral prefrontal cortex (Fellman & Van Essen, 1991). Connections from the inferior temporal cortex to dorsolateral and ventrolateral frontal areas were specified some time ago in nonhuman primates (Fellman & Van Essen, 1991; Fuster, 1985), and were recently verified in humans using probabilistic diffusion tractography (Croxson et al., 2005). Nonhuman primate and rodent (Duvernoy, 1997; Goldman-Rakic, Selemon, & Schwartz, 1984), and human evidence (Croxson et al., 2005; Powell et al., 2004) supports model connections from the HC to the dorsal and ventral frontal cortex. The HC to IT connectivity in the model represents the reciprocal connections through the perirhinal cortex, which feeds sensory information to the hippocampus via the entorhinal cortex (nonhuman and human primates, Tanaka, 1997).
2.5. Modeling Procedure
Evaluating effective connectivity quantitatively is a departure from the original application of SEM for analyzing behavioral cause-effect systems (Hayduk, 1987). Our models had bidirectional paths between regions, producing non-symmetrical feedforward and feedback dynamics (i.e., paths). This may cause estimation failure in obtaining a maximum likelihood solution, and solution instability due to the number of estimated paths. We adapted the methodological solutions proposed by McIntosh and Gonzalez-Lima (1994) to construct all SEMs. In this iterative estimation method, one direction of path coefficients (e.g., feedforward) is estimated and fixed before estimating the other direction (i.e., feedback). Models were implemented using MX32, version 1.55 (Neale, Boker, Xie, & Maes, 2003).
Starting model ROI variance parameters were fixed at 0.50. This both facilitates path estimation and constrains the proportion of activity in an area explained by exogenous brain activity and the influence of an area on itself (McIntosh, 2000). Thus, it is desirable to have variance coefficients below 1.00 in the final model. After the feedforward and feedback paths (strengths) were estimated, variances were adjusted from 0.50 to reduce over and under fit based on an inspection of the residuals from the observed and predicted correlations generated by the model. Final variance coefficients ranged between 0.02 and 0.77. The different values reflect the relative contribution of an ROI in reference to the functional anatomy of the task and the ROIs in the model network (cf. McIntosh, 2000; McIntosh & Gonzalez-Lima, 1994).
The starting value of path coefficients was 0.50 (Neale et al., 2003). The path coefficients were constrained between −1.00 and 1.00, and variances between 0.00 and 1.00. After the first, and on each successive fitting run, one feedforward path coefficient was fixed at the estimated value, starting at the connection between V1/V2 and repeated through the feedforward connections in the same manner (e.g., left to right in Figures 1 & 2). Next, feedback connections were estimated starting from DIFG and going back to V1/V2. Paths and path estimations for the Letter Strings models for both groups were adjusted using residuals. Models were reestimated (i.e., to determine stability) from different starting paths. For example, feedforward fitting began with connections between IT and HC, and estimation proceeded in the feed forward direction, looping around to the V1/V2 to V4 connections, on to feedback connection. Models were also estimated starting with the feedback connections first. Neither type of procedural change resulted in a different final model. Individual connections between two regions were inverted to insure the coefficients were unique to the direction. A lack of difference in model fit when the feedforward connection value was replaced with the feedback connection value and vice versa would indicate a problem in the robustness of the model. As expected, all sign inversions also resulted in nonsignificant model fits to the data.
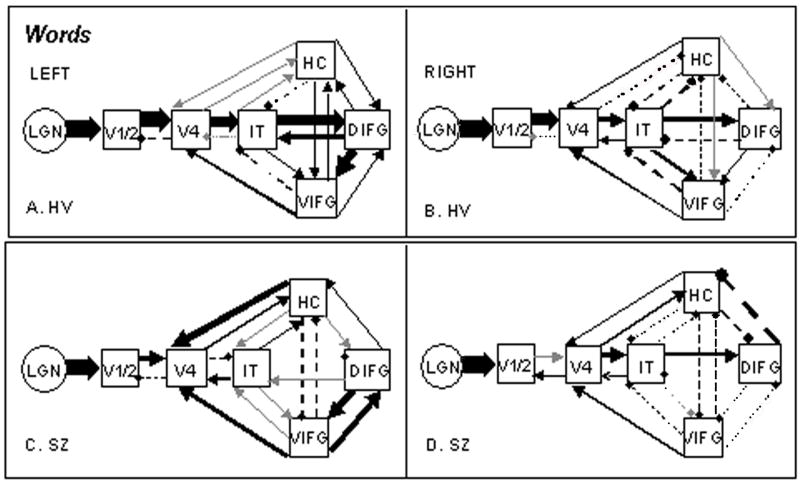
Figure 1.
Functional couplings for Words in left and right hemispheres. Solid lines represent positive coefficients; broken lines negative coefficients; gray lines, coefficients less than 0.02. Path coefficient characterization: Thin Lines = 0.00 to 0.23; Medium Lines = 0.25 to 0.39; Thick Lines = 0.46 to 0.80. For actual values see Appendix.
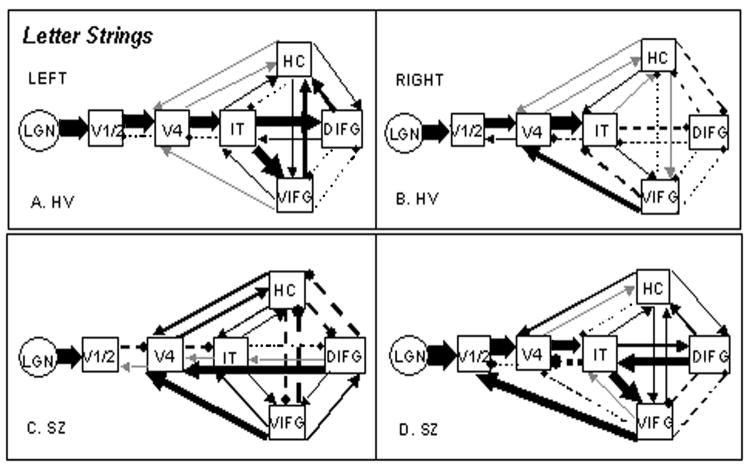
Figure 2.
Functional couplings for Letter Strings in left and right hemispheres. Solid lines represent positive coefficients; broken lines, negative coefficients; gray lines, coefficients less than 0.02. Path coefficient characterization: Thin Lines = 0.00 to 0.19:Medium Lines = 0.21 to 0.38: Thick Lines = 0.42 to 0.94. For actual values see Appendix.
3. Results
3.1. Behavioral and fMRI Subtraction
There was no difference in accuracy or RTs between groups (i.e., HV and SZ) in either condition For Words, HV accuracy was 98% and SZ accuracy was 97% (p > 0.249), and in Letter Strings both HV and SZ had accuracy of 90% (p > 0.758). Average reaction time in the words condition was 482 ms for the HV and 497 ms for the SZ (p > 0.521), while in the letter strings condition reaction times were 519 ms for the HV and 488 ms for the SZ (p > 0.130).
Table 1 shows the results of the between-group subtractions. There were no activations where the SZ exceeded the HV in either condition. On the other hand, HV had greater activation than the SZ in early visual and in visual association areas in both the Words and the Letter Strings conditions. These activations were only left-sided in Words and bilateral in Letter Strings.
3.2. Structural Equation Modeling
3.2.1. Model estimation
See Table 3 for the fit statistics for saturated (no free parameters) HV and SZ models of effective connectivity for Words and Letter Strings, by left and right hemispheres. Models were estimated such that the p-values (cf. nonfit, p ≤ 0.05) were between a lower bound of 0.10 (i.e., fit to the data) and an upper bound of 0.30 (i.e., not over-fit). The intent was to account for the regional covariance (data), but not to over-fit, and thus increase the generalizability of the observed regional interactions patterns. Model fits were evaluated with Akaike’s Information Criterion (AIC; Akaike, 1987), which should be less than zero, and Root Mean Squared Error of Approximation (RMSEA; McDonald, 1989). An RMSEA below 0.10 indicates a good fit and below 0.05 a very good fit. The AIC considers the complexity of the model with the goodness-of-fit to the sample data, and penalizes over fitting. The RMSEA is a sample size-independent measure of discrepancies in fit.
Table 3.
Fit Statistics for HV and SZ, Words and Letter Strings, Left and Right Hemispheres
| Words | ||||
|---|---|---|---|---|
| Group | HV | SZ | ||
| Hemisphere | Left | Right | Left | Right |
| Statistic | ||||
| ML χ2 Group Fit | 24.258 | 25.721 | 27.582 | 28.487 |
| p Value | 0.231 | 0.175 | 0.152 | 0.127 |
| AIC | −15.743 | −14.279 | −14.418 | −13.513 |
| RMSEA | 0.039 | 0.059 | 0.055 | 0.057 |
|
Letter Strings
| ||||
| ML χ2 Group Fit | 25.032 | 24.571 | 26.827 | 25.556 |
| p Value | 0.246 | 0.266 | 0.177 | 0.224 |
| AIC | −16.968 | −17.429 | −15.174 | −16.444 |
| RMSEA | 0.050 | 0.035 | 0.050 | 0.044 |
3.2.2. Between group model comparisons
In no comparison did the HV model provide a reliable account of SZ effective connectivity, as indicated by statistically significant differences. The Maximum Likelihood goodness-of-fit differences between models for Words in the left hemisphere was χ2diff(42) = 237.74, p < .001; for right hemisphere Words, χ2diff(42) = 77.35, p < .001; for Letter Strings left hemisphere, χ2diff(42) = 549.03, p < .001; and for right hemisphere Letter Strings, χ2diff(42) = 171.72, p < .001. For Letter Strings, the statistics reflect the difference computed on the common paths---the SZ left and right hemispheres each had one additional path than the HV models. Figure 1 illustrates Group by Hemisphere models for Words; Figure 2 illustrates the same for Letter Strings. For all model path coefficients, see the Appendix.
Appendix.
Path Coefficients Words
| Hemisphere | ||||
|---|---|---|---|---|
| Left
|
Right
|
|||
| Group | HV | SZ | HV | SZ |
| Connections
| ||||
| LGN→VI/V2 | 0.80 | 0.65 | 0.74 | 0.75 |
| V1/V2→V4 | 0.70 | 0.25 | 0.56 | 0.01 |
| ← | −0.09 | −0.04 | −0.01 | 0.06 |
| V4→IT | 0.50 | −0.08 | 0.23 | 0.25 |
| ← | 0.00 | 0.13 | 0.03 | 0.07 |
| V4→HC | 0.00 | 0.13 | −0.08 | 0.12 |
| ← | 0.00 | 0.35 | 0.11 | 0.03 |
| IT→DIFG | 0.46 | −0.04 | 0.23 | 0.15 |
| ← | 0.20 | 0.00 | −0.11 | −0.03 |
| IT→VIFG | 0.06 | 0.00 | 0.17 | 0.00 |
| ← | −0.08 | 0.00 | −0.15 | −0.06 |
| IT→HC | 0.00 | 0.05 | −0.14 | −0.05 |
| ← | −0.02 | 0.00 | −0.14 | −0.08 |
| DIFG→VIFG | 0.39 | 0.30 | 0.03 | −0.03 |
| ← | 0.10 | 0.26 | −0.02 | −0.01 |
| DIFG→HC | 0.10 | 0.05 | −0.06 | −0.23 |
| ← | 0.04 | 0.00 | 0.00 | −0.14 |
| VIFG→HC | 0.07 | −0.11 | −0.06 | −0.05 |
| ← | 0.04 | −0.11 | 0.01 | −0.07 |
| VIFG→V4 | 0.13 | 0.23 | 0.13 | 0.15 |
Note. First region name is the path origin and the second is the termination. First path value entry in the column is the path value for this path and the second is for the reciprocal path. Values were constrained between −1 and 1. Paths below 0.01 are represented by zero.
3.2.3. WORDS network connectivity
In Words, HV showed robust effective connectivity (i.e., magnitude of paths) in the dominant left hemisphere ventral path, with prevailing feedforward directionality (Figure 1A). The right hemisphere connectivity strengths in the HV reflected a generally lower level (qualitatively) of effective connectivity, with a pattern similar to the left (Figure 1B). In contrast to the HV, SZ exhibited lower-strength feedforward effective connectivity from the primary visual cortex into temporal areas, particularly in the left hemisphere connections from V4 to IT and IT to DIFG (Figure 1C). The right hemisphere SZ connectivity was qualitatively more similar to the HV right hemisphere. A notable between-group difference was the strong feedback connectivity in the left hemisphere for SZ (Figure 1C), compared to low levels of feedback in HV (Figure 1A). In particular, reciprocal SZ VIFG to DIFG connections created a stronger recurrent frontal connectivity. There was also top-down (feedback) influence from both the VIFG and HC regions on V4 in SZ.
3.2.4. LETTER STRINGS network connectivity
In Letter Strings (Figure 2), HV connectivity in both hemispheres was qualitatively similar to that of left hemisphere Words, consistent with our earlier findings that orthographic processing for both string types is more likely to occur in a graded manner and not lateralized left for words and right for letter strings (Tagamets et al., 2000b). However, a distinct feature for HV Letter Strings was strong feed-forward influence from IT to both frontal areas, in contrast to the forward effective connectivity from IT to mainly the DIFG in HV Words (Figure 1A). The SZ left hemisphere Letter Strings (Figure 2C) effective connectivity again (cf. SZ Words) lacked feedforward directionality, and the most robust couplings were those from the two frontal areas to V4, and from HC to V4. The Letter Strings model would not fit the data without the additional DIFG to V4 path (cf. HV). In contrast to the left hemisphere, the SZ right hemisphere showed the strongest effective connectivity across the network in either condition or hemisphere in the SZ group (Figures 1C, 1D and 2C, 2D). Further, the feedforward coactivity in the right hemisphere was qualitatively comparable in magnitude to HV, in contrast to the left hemisphere in Letter Strings and both hemispheres in Words, suggesting right hemisphere compensation in Letter Strings, but not in Words.
4. Discussion
We hypothesized that word and letter strings processing would reveal left hemisphere dysfunction in schizophrenia. When compared to the functional organization of HV models, SZ models showed decouplings, decreased lateralization, and decreased feedforward coupling compared to increased feedback coupling.
In the HV comparison group, we observed more lateralized left > right connectivity in the Words condition than in Letter Strings, which is consistent with prominent theories of language function (cf. Weems & Reggia, 2006). It is important to note that although left > right BOLD activations in language tasks are a relatively frequent finding in healthy comparison subjects, this does not automatically imply that connectivity would also show this pattern. Connectivity reflects the degree of coordinated activity between regions, and it is possible for weakly activated regions from a particular contrast to be strongly coupled with each other. Likewise, two strongly activated regions may not be coupled if they each receive their major inputs from separate sources. The predominantly feedforward direction of functional coupling in the HV networks for both Words and Letter Strings conditions is consistent with the task demands. A purely perceptual matching strategy is sufficient for one-back performance, whereas the semantic/linguistic nature of the Words presumably explains the left lateralization (cf. Binder et al., 2003). The lack of strong feedback suggests that these factors are likely to be automatic and do not require effortful language processes.
Subtraction Interpretation
The subtraction analyses gave the first indication that there are anomalies in basic access to linguistic information in schizophrenia. Between-group differences were found in the HV – SZ comparison, but not in SZ – HV. In Words, HV activation was greater than SZ only in the left IT/fusiform boundary area that is thought to be involved in visual word recognition (Cohen et al., 2000). The HV activations exceeded the SZ activations bilaterally in the Letter Strings condition in extrastriate cortex on the right and in early visual cortex on the left. Both groups had near perfect performance in the Words task and equivalent decrements in performance in Letter Strings, suggesting that the SZ are able to recruit linguistic attributes in support of the Words task as well as the HV. Taken together, these results suggest that deficits in SZ begin in early perceptual processes even in tasks that require minimal access to language. Our modeling results add further support for this conclusion.
Interpretation of SZ Models
Considering the similar patterns of feedforward decoupling and excessive left-hemisphere feedback connections present in both conditions for SZ, the results suggest that the left hemisphere connectivity may be dysfunctional in schizophrenia, whether in language or other cognitive domains. Because coherent language depends largely on the left hemisphere, this dysfunction is likely to be disproportionately reflected in tasks that involve language. Further, the anomalies in left hemisphere organization in the SZ models demonstrate the role of functional couplings in processing. This is in addition to the reduced or increased activity reported in schizophrenia across various stimulus paradigms (overviews, Glahn et al., 2005; Gur & Chin, 1999; Hill et al., 2004).
Contrary to the hypothesized general right hemisphere compensation for left hemisphere dysfunction, the effective connectivity was not increased in right hemisphere SZ Words. However, there was strong feedforward right hemisphere connectivity in Letter Strings. In light of the SZ left hemisphere anomalies and equivalent between-group behavioral performance, the right hemisphere contributions may not comprise increased right hemisphere activity, but rather more task adaptive activity. Our lateralization results offer some support for ideas that the right hemisphere is more involved in language function in schizophrenia (Artiges et al., 2000; Sommer et al., 2001; Sommer et al., 2004; Woodruff et al., 1997), when network effective connectivity is considered. However, hemispheric interactions may modify this conclusion on future examination, as left and right hemisphere language functions do not operate independently (cf. Crow, 1997; Mitchell & Crow, 2005). Previously, we found evidence for increased functional interactions in SZ compared to HV between homologous left and right brain regions in conditions that involve real words, but not with non-words (published abstract, Tagamets et al., 2001). If such interactions drive language functions, then the weak effective connectivity in right hemisphere SZ Words compared to strong right hemisphere effective connectivity in SZ Letter Strings supports “normal” right hemisphere contribution for stimuli with little or no semantic content. A contrary interpretation would be that the observed right hemisphere activity reflects generalized cortical inefficiency and not compensatory mechanisms. However, the differences in regional interactions between HV and SZ models were associated with equivalent performance, and a compensatory account is consistent with similar proposals regarding word encoding (cf. Wolf et al., 2007) and frontal function in schizophrenia (cf. Tan et al., 2006).
Relationship to Processing in Schizophrenia
The general posterior/anterior disconnectivity observed in SZ may result in aberrant interactions between executive and perceptual/memory functions (cf. Condray et al., 2002; Meyer-Lindenberg et al., 2005; Phillips & Silverstein, 2003). However, evidence in the current study for functional decouplings between lower (V1/V2) and higher (V4) visual areas, the visual and temporal association areas (V4 and IT), and feedforward decoupling from the frontal cortex indicates that aberrant left hemisphere activity may not be confined to temporal/frontal disconnection in basic access networks. An apparent loss of coordination between executive and sensory processing may be in part driven by aberrant sensory processing in schizophrenia, observed in both visual and auditory modalities (e.g., Butler & Javitt, 2005; Braff & Light, 2004). This results in both slower and weaker stimulus signals. Some propose encoding aberrations result in reduced stimulus signal and/or increased noise (e.g., Philips & Silverstein, 2003). Therefore, the strong top-down influences observed in the left hemisphere for both Words and Letter Strings may reflect compensatory processes, such as attention (cf. Nestor et al., 2001). Compensatory mechanisms may attenuate efficiency decreases in automatic processes, such as the identification and comparison of a current and previous stimulus (e.g., Meyer-Lindenberg et al., 2005) and orthographic processing (Tagamets et al., 2000b). Specifically, frontal feedback projections to posterior cortical areas may increase the contrast or saliency of a perceptual signal through signal amplification, as observed in nonhuman primates (De Weerd, Peralta, Desimone, & Ungerleider, 1999; Hupé et al., 1998). The model HC feedback into V4 in SZ may reflect an increased role for visual attention, possibly modulated through the V4-thalamocortical circuit thought to be specific for attention (LaBerge, Carter, & Brown, 1992). A recent study demonstrated that HC feedback also modulates input from lower visual areas during word recognition (Halgren et al., 2006).
In sum, our model network interactions indicate that, although performance may be equivalent, subtle differences in perceptual access exist in schizophrenia. These in turn may alter upstream processing when task demands change: for example, the change from the relatively automatic processes in one-back matching using words to the effortful processing of words during speech. Frontal control processes diverted to support access-related perceptual processing would be unavailable for higher-level word, sentence, and discourse processing. This type of performance effect was observed in schizophrenia (Meyer-Lindenberg et al, 2005) as a function of parametrically increasing frontal load while using the same stimuli and response mode: simple reaction, stimulus discrimination, choice reaction, continuous perceptual matching (a one-back task), and continuous delayed response (a two-back working memory task). Comparisons using RT decomposition and accuracy from each task level indicated that perceptual matching performance deficits in schizophrenia in turn affected performance in the hierarchically successive two-back matching task (Meyer-Lindenberg et al., 2005).
We suggest that in language these subtle differences in the way the regions influence each other during single word and letter string processing may predict some anomalies observed at the phrase, sentence, and discourse levels in schizophrenia (reviews; Covington et al., 2005; DeLisi, 2001; Kuperberg & Kaplan, 2003). This is a testable hypothesis for future studies; and although we evaluated networks associated with equivalent between-group accuracy, we suggest our results are relevant to previous behavioral results indicating atypically organized semantic networks in SZ. Specifically, there is reduced or slower access, as measured by the number of words produced in verbal fluency (meta analysis, Bokat & Goldberg, 2003), and reduced or enhanced semantic priming (review, Minzenberg, Ober, & Vinogradov, 2002). The latter mixed results are thought to reflect an interaction between the task dependent demand for control processes (i.e., working memory, attention, and executive functions) and the structure and functional attributes of the semantic system (Minzenberg et al., 2002). The disconnectivity, differences in lateralization, and the ratio of feedforward and feedback influences on stimulus processing we observed might also mediate word-level processing. This is in addition to two previously suggested mechanisms: the speed at which activity spreads through a semantic network and the strength and pattern of semantic associations in the network (cf. Kerns & Berenbaum, 2002; Minzenberg et al., 2002).
Interpretation Notes
It is important to note that the apparent feedforward disconnections observed in the SZ results could arise from several sources. One possibility is that there is a “real” functional disconnection, in the sense that information from sensory regions does not pass along this pathway. In this case, it would be expected that other networks, for example, homologous right-sided pathways, could play a compensatory role. Although we did not model homologous regional connections, they may modulate language function in schizophrenia (Foucher et al., 2005). Some researchers propose that the atypical functional lateralization observed in schizophrenia is the result of a left hemisphere failure to inhibit right hemisphere activity (Sommer et al., 2001). This account assumes that left hemisphere activity is normal, and not high or low, as assumed in other accounts that depend on aberrant left hemisphere activity (overviews, Glahn et al., 2005; Gur & Chin, 1999; Hill et al., 2004). Our own fMRI studies suggest a tendency in SZ for excessive functional interactions between homologous left and right brain regions in conditions that involve real words, but not with non-words (published abstract, Tagamets et al., 2001). Another interpretation of the apparent feedforward disconnection in SZ is that information does pass forward along this pathway, but the strong feedback from frontal and hippocampal regions dominates existing coordinated activity between posterior regions, thus swamping them with top-down influences. In light of the recurrent frontal activity, the strong feedback into visual areas, and weak feedforward connectivity in the left hemisphere in both stimulus conditions, we suggest that in schizophrenia there may an increased dependence on internally generated states, along with a decreased dependence on environmental input. These together may contribute to the disconnection from “reality” observed in schizophrenia.
There are also some qualifications to our modeling results. The sign of the path coefficients are not interpretable in terms of increases or decreases in activity. The BOLD signal reflects excitatory and inhibitory synaptic activity, plus modulatory neurotransmitter effects. For example, inhibition is thought to increase local blood flow (fMRI signal) even if underlying neuronal activity decreases (Tagamets & Horwitz, 2001). Thus a positive connection weight in an SEM cannot be interpreted as an excitatory underlying connection. Our previous modeling results suggest that the interaction of local circuitry and the context of converging excitatory and inhibitory neuronal activity is likely to influence imaging data (Tagamets & Horwitz, 2001; Winder et al., 2007). Additionally, our SEM models did not account for the nonlinear nature of inter-regional brain connections or local circuitry. The introduction of nonlinear terms with asymmetric values in feedforward and feedback paths would create undesirable fitting problems. Finally, although the effective connectivity results for the SZ and HV are largely consistent with the functional connectivity results cited in the introduction, the generalizability of our results should be verified in larger samples.
Conclusion
The observed strong feedforward couplings in the left hemisphere ventral stream, and between the inferior temporal and lateral frontal regions, corroborates and extends previous functional connectivity results for language tasks using healthy comparison subjects. In contrast, for persons with schizophrenia, the lack of regional couplings in the ventral stream, and specifically, the lack of coupling in the feedforward direction, indicates that left hemisphere dysfunction is present even in basic access for linguistic stimuli. Potentially compensatory mechanisms included increased right hemisphere contributions and top-down influence from frontal regions into posterior regions. Due to the hierarchical organization of the cortex, where lower level processing affects successive higher level processes (e.g., Felleman & Van Essen, 1991), the anomalies we observed in network function during automatic linguistic access may affect language in schizophrenia. This would be in addition to anomalies found in higher order semantic networks and executive functions (cf. reviews, Goldberg et al., 1998; Kerns & Berenbaum, 2002).
Path Coefficients Letter Stings
| Hemisphere | ||||
|---|---|---|---|---|
| Left
|
Right
|
|||
| Group | HV | SZ | HV | SZ |
| Connections
| ||||
| LGN→VI/V2 | 0.77 | 0.71 | 0.73 | 0.94 |
| V1/V2→V4 | 0.62 | −0.12 | 0.43 | 0.70 |
| ← | −0.03 | 0.00 | 0.02 | −0.14 |
| V4→IT | 0.52 | −0.13 | 0.63 | 0.45 |
| ← | −0.04 | −0.01 | −0.04 | −0.28 |
| V4→HC | 0.00 | 0.17 | 0.00 | 0.00 |
| ← | 0.00 | 0.15 | 0.00 | 0.16 |
| IT→DIFG | 0.42 | −0.05 | −0.09 | 0.17 |
| ← | 0.08 | 0.00 | −0.05 | 0.35 |
| IT→VIFG | 0.48 | 0.09 | 0.09 | 0.43 |
| ← | 0.06 | 0.12 | −0.14 | 0.01 |
| IT→HC | 0.10 | 0.09 | −0.01 | 0.03 |
| ← | −0.03 | 0.12 | 0.03 | −0.03 |
| DIFG→VIFG | −0.01 | 0.07 | −0.02 | −0.10 |
| ← | −0.01 | 0.11 | −0.02 | −0.09 |
| DIFG→HC | 0.23 | −0.18 | −0.06 | 0.16 |
| ← | 0.05 | −0.18 | −0.10 | 0.11 |
| VIFG→HC | 0.19 | −0.22 | −0.06 | 0.13 |
| ← | 0.06 | −0.15 | −0.01 | 0.03 |
| VIFG→V4 | 0.01 | 0.38 | 0.25 | −0.09 |
| VIFG→V1/V2 | 0.41 | |||
| DIFG→V4 | 0.34 | |||
Note. First region name is the path origin and the second is the termination. First path value entry in the column is the path value for this path and the second is for the reciprocal path. Values were constrained between −1 and 1. Paths below 0.01 are represented by zero.
Acknowledgments
This research was supported by NIMH grant T32MH067533 to Maryland Psychiatric Research Center (J. A. G.) and by NIMH grant K01MH064622 to Malle-Anne Tagamets (M-A. T. & C. R. C.). Additional funding came from the Advanced Center for Intervention and Services Research Grant P30 MH06850 NIMH, National Institutes of Health (NIH), and University of Maryland General Clinical Research Center Grant M01 RR 165001, National Center for Research Resources (NCRR), NIH. The authors thank four anonymous referees for their helpful comments on an earlier version of this paper. We would like also to thank the Schizophrenia Related Disorders Program at MPRC for providing patient volunteers, and express our gratitude to all those who gave their time and effort to participate in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Artiges E, Martinot JL, Verdys M, Attar-Levy D, Mazoyer B, Tzourio N, Giraud MJ, Paillere-Martinot ML. Altered hemispheric functional dominance during word generation in negative schizophrenia. Schizophrenia Bulletin. 2000;26:709–721. doi: 10.1093/oxfordjournals.schbul.a033488. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Bokat CE, Goldberg TE. Letter and category fluency in schizophrenic patients: A meta-analysis. Schizophrenia Research. 2003;64:73–78. doi: 10.1016/s0920-9964(02)00282-7. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Boksman K, Théberge J, Williamson P, Drost DJ, Malla A, Densmore M, Takhar J, Pavlosky W, Menon RS, Neufeld RWJ. A 4.0-T fMRI study of brain connectivity during word fluency in first-episode schizophrenia. Schizophrenia Research. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology. 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Brannen JH, Badie B, Moritz CH, Quigley M, Meyerand ME, Haughton VM. Reliability of functional MR imaging with word-generation tasks for mapping Broca’s area. American Journal of Neuroradiology. 2001;22:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Current Opinions in Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR, van Kammen DP, Kasparek A. The language system in schizophrenia: Effects of capacity and linguistic structure. Schizophrenia Bulletin. 2002;28:475–490. doi: 10.1093/oxfordjournals.schbul.a006955. [DOI] [PubMed] [Google Scholar]
- Covington MA, He C, Brown C, Naçi L, McClain JT, Fjordbak BS, Semple J, Brown J. Schizophrenia and the structure of language: The linguist’s view. Schizophrenia Research. 2005;77:85–98. doi: 10.1016/j.schres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as a failure of hemispheric dominance for language. Trends in Neuroscience. 1997;20:339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TEJ, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MFS. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. The Journal of Neuroscience. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE. Speech disorder in schizophrenia: Review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophrenia Bulletin. 2001;27:481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Peralta MR, Desimone R, Ungerleider LG. Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nature Neuroscience. 1999;2:753–758. doi: 10.1038/11234. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus. 2. New York: Springer; 1997. [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fiez J. Phonology, semantics and the role of the left inferior prefrontal cortex. Human Brain Mapping. 1997;5:79–83. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. (SCID-I/P; SCID-I/NP) [Google Scholar]
- Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Vidailhet P, Chanraud S, Gounot D, Grucker D, Pins D, Damsa C, Danion JM. Functional integration in schizophrenia: Too little or too much? Preliminary results on fMRI data. Neuroimage. 2005;26:374–378. doi: 10.1016/j.neuroimage.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Fuster J. The prefrontal cortex and temporal integration. In: Peters A, Jones EG, editors. Cerebral cortex, Vol. 4: Association and auditory cortices temporal integration. New York and London: Plenum Press; 1985. pp. 151–177. [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR. Cognitive Substrates of Thought Disorder, I: The semantic system. American Journal of Psychiatry. 1998;155:1671–1676. doi: 10.1176/ajp.155.12.1671. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gur RE, Chin S. Laterality in functional brain imaging studies of schizophrenia. Schizophrenia Bulletin. 1999;25:141–156. doi: 10.1093/oxfordjournals.schbul.a033361. [DOI] [PubMed] [Google Scholar]
- Halgren E, Wang C, Schomer DL, Knake S, Marinkovic K, Wu J, Ulbert I. Processing stages underlying word recognition in the anteroventral temporal lobe. Neuroimage. 2006;30:1401–1413. doi: 10.1016/j.neuroimage.2005.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayduk LA. Structural Equation Modeling with LISREL. Baltimore, MD: The Johns Hopkins University Press; 1987. [Google Scholar]
- Hill K, Mann L, Laws KR, Stephenson CME, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: A meta-analysis of functional imaging studies. Acta Psychiatrica Scandinavica. 2004;110(4):243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Scheef L. Mediotemporal contributions to semantic processing: fMRI evidence from ambiguity processing during semantic context verification. Hippocampus. 2005;15:597–609. doi: 10.1002/hipo.20080. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Braun AR. Brain network interaction in auditory, visual and linguistic processing. Brain and Language. 2004;89:377–384. doi: 10.1016/S0093-934X(03)00349-3. [DOI] [PubMed] [Google Scholar]
- Hupé JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. Cortical feedback improves discrimination between figure and background by V1, V2, and V3 neurons. Nature. 1998;394:784–797. doi: 10.1038/29537. [DOI] [PubMed] [Google Scholar]
- Jaynes J. The Origin of Consciousness in the Breakdown of the Bicameral Mind. Boston: Houghton Mifflin; 1977. [Google Scholar]
- Jennings JM, McIntosh AR, Kapuv S, Zipursky RB, Houle S. Functional network difference in schizophrenia: A rCBF study of semantic processing. Neuroreport. 1998;9(8):1697–1700. doi: 10.1097/00001756-199806010-00005. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. Journal of Abnormal Psychology. 2002;111:211–224. [PubMed] [Google Scholar]
- Kim JJ, Ho Seok J, Park HJ, Soo Lee D, Chul Lee M, Soo Kwon J. Functional disconnection of the semantic networks in schizophrenia. Neuroreport. 2005;16(4):355–59. doi: 10.1097/00001756-200503150-00010. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Caplan D. Language dysfunction in schizophrenia. In: Schiffer RB, Rao SM, Fogel BS, editors. Neuropsychiatry. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 444–466. [Google Scholar]
- LaBerge D, Carter M, Brown V. A network simulation of thalamic circuit operations in selective attention. Neural Computation. 1992;4:318–331. [Google Scholar]
- Lawrie SM, Büchel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced fronto-temporal functional connectivity in schizophrenia associated with auditory hallucinations. Biological Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- McDonald RP. An index of goodness-of-fit based on noncentrality. Journal of Classification. 1989;6:97–103. [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Networks. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Human Brain Mapping. 1994;2:2–22. [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of regional neuronal interaction. Journal of Cognitive Neuroscience. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. American Journal of Psychiatry. 2005;162:1206–1208. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Ober BA, Vinogradov S. Semantic priming in schizophrenia: A review and synthesis. Journal of the International Neuropsychological Society. 2002;8:699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- Mitchell RLC, Crow TJ. Right hemisphere language functions and schizophrenia: The forgotten hemisphere. Brain. 2005;128:963–978. doi: 10.1093/brain/awh466. [DOI] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PCM. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magnetic Resonance in Medicine. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. MX: Statistical Modeling. 6 Richmond, VA 23298: Department of Psychiatry; 2003. VCU Box 900126. [Google Scholar]
- Neely JH. Semantic priming in visual word recognition: A selective review of current theories and findings. In: Besner B, Humphries G, editors. Basic processes in reading: Visual word recognition. Hillsdale, NJ: Erlbaum; 1991. pp. 264–336. [Google Scholar]
- Nestor PG, Han SD, Niznikiewicz M, Salisbury D, Spencer K, Shenton ME, Carley RW. Semantic disturbance in schizophrenia and its relationship to the cognitive neuroscience of attention. Biological Psychology. 2001;57:23–46. doi: 10.1016/s0301-0511(01)00088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fiez JA. The processing of single words studied with positron emission tomography. Annual Reviews Neuroscience. 1993;16:509–530. doi: 10.1146/annurev.ne.16.030193.002453. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured interview for DSM-IV personality. Washington, DC: American Psychiatric Press, Inc.; 1997. [Google Scholar]
- Phillips WA, Silverstein SM. Impaired cognitive coordination in schizophrenia: Convergence of neurobiological and psychological perspectives. Behavioral and Brain Sciences. 2003;26:65–82. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Powell HWR, Guye M, Parker GJM, Symms MR, Boulby P, Koepp MJ, Barker GJ, Duncan JS. Noninvasive in vivo demonstration of the connections of the human parahippocampal gyrus. Neuroimage. 2004;22:740–747. doi: 10.1016/j.neuroimage.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Price CJ, Indefrey P, van Turennout M. The neural architecture underlying the processing of written and spoken word forms. In: Brown CM, Hagoort P, editors. The neurocognition of language. New York: Oxford University Press; 1999. pp. 211–240. [Google Scholar]
- Price CJ, Winterburn D, Giraud AL, Moore CJ, Noppeney U. Cortical localization of the visual and auditory word form areas: A reconsideration of the evidence. Brain and Language. 2003;86:272–286. doi: 10.1016/s0093-934x(02)00544-8. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliot M, Kohler C, Siegel S, Kanes S, Gur RR. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. American Journal of Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Aleman A, Ramsey N, Bouma A, Kahn R. Handedness, language lateralisation and anatomical asymmetry in schizophrenia. British Journal of Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Ramsey NF, Mandl RCW, Van Oel CJ, Kahn RS. Language activation in monozygotic twins discordant for schizophrenia. British Journal of Psychiatry. 2004;184:128–135. doi: 10.1192/bjp.184.2.128. [DOI] [PubMed] [Google Scholar]
- Tagamets M-A, Horwitz B. Integrating electrophysiological and anatomical experimental data to create a large-scale model that simulates a delayed match-to-sample human brain imaging study. Cerebral Cortex. 1998;8:310–320. doi: 10.1093/cercor/8.4.310. [DOI] [PubMed] [Google Scholar]
- Tagamets MA, Chalmers ML, Horwitz B, Bokde AL, Friedman RB. Changes in functional connectivity as a function of letterstring familiarity. Neuroimage. 2000;11:S438. [Google Scholar]
- Tagamets MA, Novick JM, Chalmers ML, Friedman RB. A parametric approach to orthographic processing in the brain: An fMRI study. Journal of Cognitive Neuroscience. 2000;12:281–297. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- Tagamets MA, O’Donnell PS, Medoff DR, Schweitzer JB, Holcomb HH. An fMRI study of single word reading in schizophrenia. Society for Neuroscience Abstracts. 2001;27:110.14. [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan M, Weinberger DR, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. American Journal of Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Mechanisms of visual object recognition: Monkey and human studies. Current Opinion in Neurobiology. 1997;7:523–529. doi: 10.1016/s0959-4388(97)80032-3. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Weems SA, Reggia JA. Simulating single word processing in the classic aphasia syndromes based on the Wernicke-Lichtheim-Geschwind theory. Brain and Language. 2006;98:291–309. doi: 10.1016/j.bandl.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Winder R, Cortes CR, Reggia JA, Tagamets M-A. Functional connectivity in fMRI: A modeling approach for estimation and for relating to local circuits. Neuroimage. 2007;34:1093–1107. doi: 10.1016/j.neuroimage.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Gur RC, Valdez JN, Loughead J, Elliot MA, Gur RE, Ragland JD. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Research. 2007;154:221–231. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff PWR, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SCR, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: A functional magnetic resonance imaging study. The American Journal of Psychiatry. 1997;154:1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC. PET studies of phonetic processing of speech: Review, replication, and reanalysis. Cerebral Cortex. 1996;6:21–30. doi: 10.1093/cercor/6.1.21. [DOI] [PubMed] [Google Scholar]