Abstract
Despite the central physiological function of the myogenic response, the underlying signalling pathways and the identity of mechanosensors in vascular smooth muscle (VSM) are still elusive. In contrast to present thinking, we show that membrane stretch does not primarily gate mechanosensitive transient receptor potential (TRP) ion channels, but leads to agonist-independent activation of Gq/11-coupled receptors, which subsequently signal to TRPC channels in a G protein- and phospholipase C-dependent manner. Mechanically activated receptors adopt an active conformation, allowing for productive G protein coupling and recruitment of β-arrestin. Agonist-independent receptor activation by mechanical stimuli is blocked by specific antagonists and inverse agonists. Increasing the AT1 angiotensin II receptor density in mechanically unresponsive rat aortic A7r5 cells resulted in mechanosensitivity. Myogenic tone of cerebral and renal arteries is profoundly diminished by the inverse angiotensin II AT1 receptor agonist losartan independently of angiotensin II (AII) secretion. This inhibitory effect is enhanced in blood vessels of mice deficient in the regulator of G-protein signalling-2. These findings suggest that Gq/11-coupled receptors function as sensors of membrane stretch in VSM cells.
Keywords: AT1 receptor, mechanotransduction, smooth muscle cell, transient receptor potential
Introduction
The mechanisms underlying the translation of mechanical stimuli into biochemical information have a fundamental function in physiology and pathophysiology, but are only poorly understood (Kung, 2005; Orr et al, 2006). Local blood flow is dynamically regulated to match the metabolic demand of peripheral tissues and organs. More than 100 years ago, Bayliss made the seminal observation that small-resistance arterial blood vessels have the intrinsic property to constrict in response to rises in intraluminal pressure (Bayliss effect). As disruption of the endothelium does not impair pressure-induced myogenic vasoconstriction, it is now assumed that myogenic responsiveness is an inherent property of vascular smooth muscle (VSM) and that it can be fine-tuned by endothelial and neurohumoral factors (Davis and Hill, 1999; Murphy et al, 2002) acting at G protein-coupled receptors (Pierce et al, 2002). The vasomotor response is of prime physiological relevance because it determines basal vascular tone and peripheral vascular resistance and regulates capillary hydrostatic pressure and organ perfusion. Consequently, impaired myogenic responsiveness and blood flow autoregulation are encountered in various pathological states, such as systemic hypertension, diabetes mellitus and stroke.
However, the underlying signalling pathways and the molecular identity of mechanosensors in VSM are largely unknown. Increased intravascular pressure causes depolarization of the arterial myocyte cell membrane (Davis and Hill, 1999), thereby activating voltage-dependent L-type Ca2+ channels (Cavs) (Moosmang et al, 2003). However, pressure-induced depolarization is not affected by L-type Ca2+ channel blockers (Knot and Nelson, 1995), implying that another stretch-activated ion channel is responsible for smooth muscle cell (SMC) depolarization.
The mechanisms linking mechanical stimuli to ion channel activation appear to rely critically on biochemical signalling cascades. There is a compelling body of evidence that phospholipase C (PLC) activation is a prerequisite for pressure-induced myogenic vasoconstriction (Thorneloe and Nelson, 2005; Inoue et al, 2006). However, the mechanism of PLC activation in response to mechanical stimuli is still elusive. As classical vasoconstrictors such as angiotensin II (AII) or endothelin exert their action by activating the PLC pathway, it is tempting to speculate that intravascular pressure and receptor agonists may engage similar signalling cascades leading to smooth muscle contraction.
A number of proteins, including stretch-sensitive ion channels, cell adhesion proteins, the cytoskeleton, receptors, G proteins, enzymes and the phospholipid bilayer of the plasma membrane itself, have been discussed as potential mechanosensors (Martinac, 2004; Kung, 2005; Ingber, 2006; Orr et al, 2006). During the past decade, a large portfolio of ion channels has been shown to make crucial contributions to the regulation of smooth muscle contractility (Beech et al, 2004; Thorneloe and Nelson, 2005). Of late, several members of the transient receptor potential (TRP) family of cation channels (Montell et al, 2002; Clapham, 2003) have been proposed as mechanosensitive ion channels, for instance, TRPC6 (Spassova et al, 2006).
However, in TRPC6-deficient mice, the Bayliss effect in cerebral arteries is not affected (Dietrich et al, 2005), proving in vivo that TRPC6 per se is not required for pressure-induced vasoconstriction. In view of these contradictory findings, we investigated the mechanism of mechanosensation and TRPC channel activation in VSM.
Results
TRPC6 is not a classical mechanosensitive ion channel
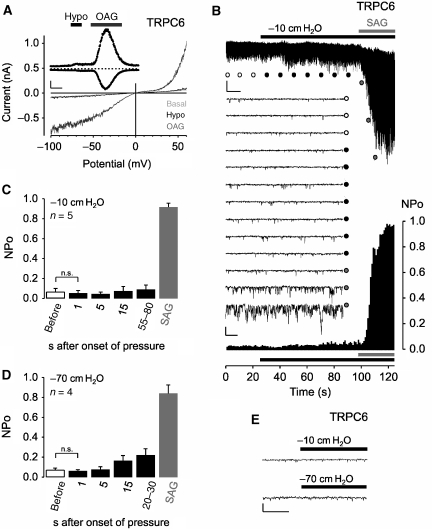
To investigate whether TRPC6 channels are mechanosensitive, the effect of osmotically induced membrane stretch (250 mOsm kg−1) on TRPC6-expressing HEK293 cells was monitored. By applying the whole-cell patch-clamp recording technique, rapidly developing transient outward and inward currents at holding potentials of ±60 mV were observed only in response to application of the membrane-permeable DAG analogue 1-oleoly-2-acetyl-sn-glycerol (OAG; 100 μM) demonstrating the functional expression of TRPC6, but they were not elicited by hypotonicity (Figure 1A). The current–voltage (IV) relations revealed functional hallmarks of the TRPC3/6/7 subfamily (Hofmann et al, 1999), that is, dual inward and outward rectification, a rise in current fluctuations with increasing driving force and a reversal potential close to 0 mV.
Figure 1.
TRPC6 per se is not mechanosensitive. (A) Whole-cell recordings from HEK293 cells expressing TRPC6; application of hypotonic stimulus (250 mOsm kg−1), ‘Hypo', and of 100 μM OAG are indicated. Current time courses at ±60 mV, zero current levels (stippled lines), time scale bar (50 s) and current scale bar (200 pA). (B–E) Single-channel recordings in inside-out patches with negative pipette pressure and subsequent SAG bath application. (B) Original current trace, scale bar (2 pA, 10 s) (top); 13 consecutive traces on an expanded time-scale at the indicated time points, scale bar (5 pA, 20 ms) (middle); the respective graph of consecutive open probabilities (NPo) in 1-s steps (bottom) is displayed. (C, D) Analysis of mean NPo values. (E) Representative expanded current traces around the time point of pressure application, scale bar (5 pA, 10 ms).
To further investigate the potential mechanosensitivity of TRPC6 in response to direct membrane stretch, we used isolated inside-out membrane patches from TRPC6-expressing HEK293 cells. Pressure application of −10 cm H2O through the patch pipette failed to activate ion currents at a holding potential of −60 mV, whereas subsequent addition of 1-stearoyl-2-arachidonoyl-sn-glycerol (SAG; 10 μM) to the bath evoked a marked increase in TRPC6 channel activity (Figure 1B). NPo values, the product of channel number and open probability, between 0.044 and 0.089 with negative pressure applied (Figure 1C) but before SAG addition, reflect a low basal channel activity of around 10%. In these experiments, the concentration of Cl− ions was reduced to 10 mM, and 50 μM 5-nitro-2-(3-phenyl-propylamino)-benzoate (NPPB) was present in the bath solution. Without these measures, the pressure stimulus promptly gated stretch-activated Cl− channels characterized by a large conductance of approximately 70 pS and amplitudes of 4–5 pA at −60 mV. Increasing the negative pipette pressure up to −70 cm H2O did not trigger a significant increase in TRPC6 activity after 1, 5 or 15 s of mechanical stimulation (Figure 1D). In some membrane patches, a slow saturable increase in NPo values up to 0.35 was observed at later time points (Figure 1D and Supplementary Figure S1), but instantaneous channel activation during the first few milliseconds was never observed (Figure 1E), suggesting that TRPC6 channels per se are not mechanosensitive.
Ligand-independent activation of AT1R elicits TRPC6-dependent cation currents
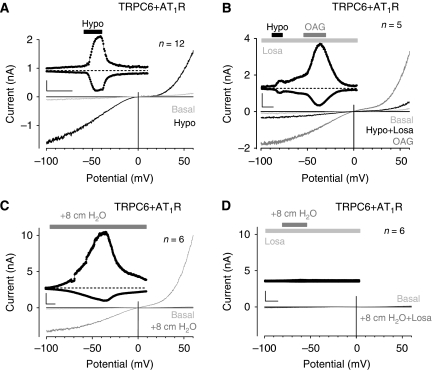
It has been reported that mechanical stress can activate the AT1 receptor (AT1R) independently of AII (Zou et al, 2004), leading to an active receptor conformation (Yasuda et al, 2008). Therefore, we co-expressed TRPC6 together with the AT1R in HEK293 cells. Osmotically induced membrane stretch invariably activated TRPC6-dependent cation currents (Figure 2A). The hypotonicity-induced TRPC6 activation could be completely suppressed by a dominant-negative TRPC6 mutant (Hofmann et al, 2002) (Supplementary Figure S2). Interestingly, the currents were substantially reduced in the presence of the selective inverse agonists losartan (1 μM) (Figure 2B) or candesartan (100 nM) (data not illustrated). Subsequent addition of OAG in the presence of the inverse agonists directly activated TRPC6 currents. As AII is not synthesized in HEK293 cells (Zou et al, 2004), these observations imply ligand-independent activation of the AT1R by membrane stretch.
Figure 2.
Indirect TRPC6 activation by membrane stretch through AT1R. Whole-cell recordings from transiently AT1R (A, B) or AT1R-Venus (C, D) and TRPC6 co-expressing HEK293 cells. (A–D) IV relationships before ‘Basal' and during hypotonicity (A, B) and direct membrane stretch (C, D) with and without 1 μM losartan, ‘Losa', are displayed; current time courses at ±60 mV, zero current levels (stippled lines), time scale bars: 50 s (A, B), 100 s (C, D) and current scale bars: 500 pA (A, B), 2 nA (C, D).
Hypotonic cell swelling may trigger additional unspecific effects apart from exerting membrane stretch by increasing the cell volume. When applying a more direct form of membrane stretch, that is, positive pipette pressure, TRPC6-dependent cation currents were readily detectable (Figure 2C). However, in the presence of losartan, TRPC6 activation by positive pipette pressure was completely abrogated (Figure 2D). These observations strongly argue for an indirect activation of TRPC6 by membrane stretch.
Mechanical activation is a common feature of many Gq/11-coupled receptors
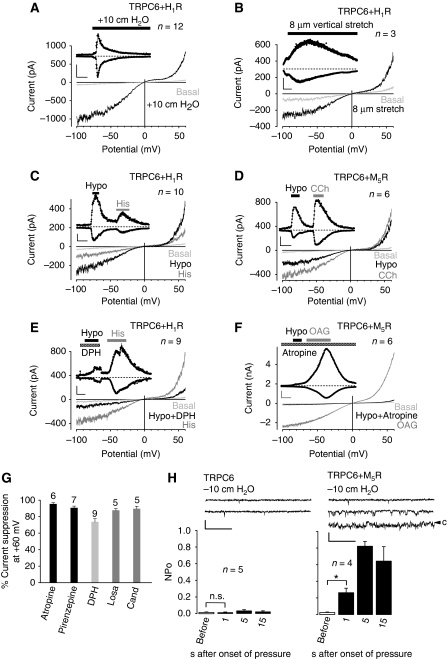
We performed similar experiments with cells co-expressing the H1 histamine receptor (H1R) and TRPC6. These cells responded to direct membrane stretch exerted by positive pipette pressure (Figure 3A) and as a second direct mechanical stimulus to vertical membrane stretch (Figure 3B) with enhanced TRPC6 currents. Hypotonic stimulation also activated TRPC6 currents (Figure 3C), and subsequent receptor activation by histamine (100 μM) elicited a second TRPC6 activation serving as an expression control. Similar results were obtained when the muscarinic receptor (M5R) was co-expressed (Figure 3D). Similar to AII, histamine or acetylcholine are not endogenously produced in HEK293 cells, indicating that the observed receptor activation is ligand independent. In both cases, membrane stretch-induced currents were strongly reduced by the application of diphenhydramine (100 μM) and atropine (1 μM), respectively (Figure 3E and F).
Figure 3.
Inverse agonists and antagonists prevent mechanical activation of GPCRs. Whole-cell measurements of H1R (A–C, E) or M5R 100 s (D, F) with stimulation by positive pipette pressure (A), with vertical stretch (B) or with hypotonicity (C–F) in the presence or absence of 100 μM diphenhydramine, ‘DPH' (E) or 1 μM atropine (F). Agonist stimulation was performed with 100 μM histamine, ‘His' or with 100 μM carbachol ‘CCh'. Direct TRPC6 stimulation was performed with 100 μM OAG. Current time courses at ±60 mV, zero current levels (stippled lines), scale bars: 500 pA (A), 200 pA (B–E) or 1 nA (F) and 50 s (A, C–F) or 100 s (B). (G) Comparison of TRPC6 current suppression by atropine (1 μM), pirenzepine (1 μM), diphenhydramine (100 μM), losartan (1 μM) and candesartan, ‘Cand', (100 nM) with hypotonic stimulation of HEK293 cells co-expressing TRPC6 and the respective receptors. (H) Analysis of NPo values in cell-attached patches. (H) (top) Representative current traces before, during the first (left and right) and the fifths (right) second after application of −10 cm H2O pipette pressure. The closed state is indicated with ‘c' (right), scale bars (5 pA, 20 ms).
To compare the inhibitory effects of antagonists or inverse agonists used, we transiently expressed a select array of Gq/11-coupled receptors, that is, M5R, H1R and AT1R and monitored suppression of TRPC6 currents at +60 mV elicited by osmotically induced membrane stretch (Figure 3G). Current reductions were normalized to maximal currents in response to either OAG (M5R and AT1R) or histamine (H1R). At maximally effective concentrations, the various compounds tested reduced cation currents by 72–95%. As the chemical nature of the substances is very diverse, direct unspecific effects on TRPC proteins are unlikely.
In addition, cells transiently expressing other vasopressor receptors, ETA endothelin (ETAR) or V1A vasopressin receptors (V1AR), responded to hypotonic bath solutions with TRPC6 activation that was inhibitable by the selective antagonist darusentan in the case of ETAR (Supplementary Figure S3). A comparison between different Gq/11-coupled receptors revealed the following order of mechanosensitivity: H1R>AT1R>M5R>V1AR (Supplementary Figure S4). On the contrary, we did not obtain any evidence for mechanosensitivity of Gs-coupled receptors as exemplified by the β2-adrenergic receptor (Supplementary Figure S5).
Using the cell-attached patch-clamp configuration, which preserves intracellular signalling pathways, only co-expression of GPCRs, that is, M5Rs (Figure 3H) or AT1Rs (data not illustrated) with TRPC6 resulted in an indirect channel activation by membrane stretch. Altogether, these observations strongly argue against a direct activation of TRPC6 by membrane stretch.
Notably, TRPC6 could be replaced by TRPC3 or TRPC7, which were similarly activated by hypotonicity and receptor stimulation (Supplementary Figure S6), indicating that all DAG-sensitive TRPC channels are indirectly sensitive to membrane stretch. In addition to electrophysiological measurements, we corroborated our findings using single-cell fluorescence imaging of fura-2-loaded intact cells (Supplementary Figure S7).
TRPC6 activation by membrane stretch depends on G proteins and PLC activation
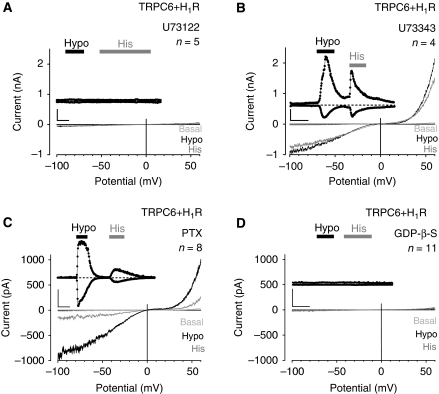
Next, we examined the intracellular signalling cascade from mechanically stimulated receptor to channel activation. In H1R and TRPC6 co-expressing HEK293 cells, the PLC inhibitor U73122 prevented TRPC6 activation in response to membrane stretch and agonist challenge of the H1R (Figure 4A), whereas cells promptly reacted to both kinds of stimuli after addition of the inactive analogue U73343 (Figure 4B).
Figure 4.
Osmotically induced membrane stretch activates phospholipase C and G proteins. (A–D) Whole-cell recordings from HEK293 cells co-expressing TRPC6 and H1R. Recording with 10 μM U73122 (A) or with 10 μM U73343 (B) in the pipette solution. Recordings of cells pretreated with 100 ng ml−1 PTX for 16 h (C) or with 2 mM GDP-β-S in the pipette solution (D). IV relationships before ‘Basal', during hypotonic stimulation, ‘Hypo' and receptor stimulation with 100 μM histamine, ‘His' are shown. (A–D) Insets show current time courses at ±60 mV, hypotonic and histamine stimulations, zero current levels (stippled lines); scale bars: 500 pA, 50 s.
PLC-β isoforms can be stimulated by GTP-loaded Gαq/11 subunits or by Gβγ dimers released from activated Gi/o proteins (Rhee, 2001). Pertussis toxin (PTX) pretreatment of HEK293 cells co-expressing the H1R and TRPC6 to uncouple Gi/o proteins from receptors did not affect TRPC6 activation by hypotonic cell swelling or agonist challenge (Figure 4C).
To demonstrate a participation of G proteins, the non-hydrolysable GTP analogue GDP-β-S was included in the pipette solution. In the presence of GDP-β-S, ionic currents triggered by hypotonic cell swelling or by the addition of histamine were completely abolished (Figure 4D), suggesting that Gq/11-type G proteins mediate membrane stretch-induced PLC and subsequent TRPC6 activation. Notably, membrane stretch-induced TRPC6 activation is independent of store depletion as induced by cyclopiazonic acid (Supplementary Figure S8).
Apart from GPCRs, TRPC channels can also be engaged by receptor tyrosine kinases. However, we observed that tyrosine kinase activation does not have an important function for TRPC6 activation by osmotically induced membrane stretch in HEK293 cells (Supplementary Figure S9).
Mechanically induced active receptor conformations are prevented by inverse agonists
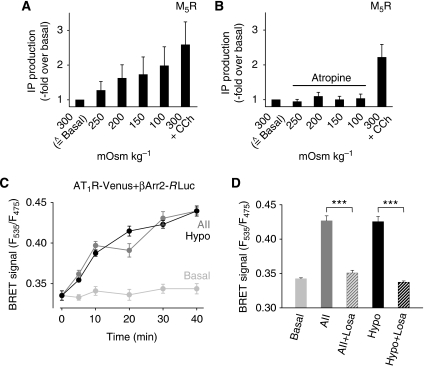
COS-7 cells transiently expressing the M5R reacted to stepwise reductions of medium osmolality with increased agonist-independent inositol phosphate formation (Figure 5A). Receptor occupation by atropine completely prevented inositol phosphate formation triggered by hypotonic stimuli, whereas muscarinic receptors were efficiently activated by carbachol in the absence of atropine (Figure 5B). Similarly, H1R-expressing COS-7 cells reacted to a decrease in osmolality with increased inositol phosphate production (data not shown).
Figure 5.
Active receptor conformations induced by membrane stretch. (A, B) Inositol phosphate (IP) accumulation as measured in one out of 3 representative assays with M5R expressing COS-7 cells. Agonist stimulation with 100 μM carbachol, ‘CCh', is also displayed. (C, D) BRET1 assay with COS-7 cells co-expressing AT1R-Venus and βArr2-Rluc. (C) Representative time courses of BRET signals determined as quadruplets. (D) Summary of three independent BRET assays. After 30 min, BRET signals elicited by stimulation with an isotonic solution, ‘Basal', 100 nM AII, with a hypotonic solution, ‘Hypo' (273 mOsm kg−1), and under 1 μM losartan, ‘Losa'.
To gather evidence for membrane stretch-induced conformational changes in the receptor, we applied the bioluminescence resonance energy transfer (BRET) technique and co-expressed eYFP (Venus)-fused AT1R and β-arrestin-2 covalently linked to Renilla luciferase (Rluc) in COS-7 cells. Hypotonic cell swelling, as well as receptor activation at 22°C, led to a marked increase in the BRET signal (Figure 5C). Conformational changes in the receptor as evidenced by β-arrestin-2 recruitment in response to both stimuli were abrogated by preincubation with losartan (Figure 5D). Membrane stretch recruits β-arrestins to the receptor as efficiently as maximal stimulation by agonist, demonstrating that membrane stretch results in an active receptor conformation, which can be precluded by previous inverse agonist binding.
Expression of AT1R imparts mechanosensitivity to aortic SMCs
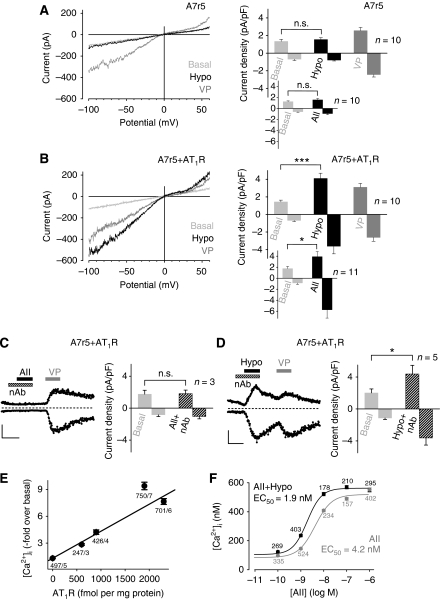
A7r5 cells are derived from embryonic rat thoracic aorta, an elastic vessel not developing myogenic tone. Rapidly developing TRPC6-like currents were observed only in response to receptor activation by vasopressin (Figure 6A) but not in response to osmotically induced membrane stretch (Jung et al, 2002). In the aorta (Nickenig et al, 1998) devoid of myogenic responsiveness, AT1R density is around 5 fmol mg−1, whereas it amounts to around 200 fmol mg−1 in the afferent arterioles (Ruan et al, 1997). As AT1R are sparsely expressed in A7r5 cells, addition of AII did not affect TRPC6 activity (Figure 6A, right, inset). However, after increasing AT1R density in A7r5 cells, hypotonic bath solutions caused highly significant TRPC6-like currents (Figure 6B) as did the addition of exogenous AII (Figure 6B, right, inset). Basal or vasopressin-induced current densities of untransfected and transfected A7r5 cells were not significantly different (Figure 6A and B). Ligand-binding studies revealed an AT1R density of 88 fmol mg−1 in native A7r5 cells, whereas an A7r5 cell line stably expressing AT1R had a 15-fold higher receptor density of 1305 fmol mg−1. In both cell lines, the vasopressin receptor (V1AR) density was around 118 fmol mg−1. Furthermore, the number of functional TRPC6 channels per cell in untransfected as well as in AT1R overexpressing A7r5 cells (approximately 360) was comparable with the number of channels in transfected HEK293 cells (approximately 470).
Figure 6.
Expression of AT1R leads to mechanosensitivity of aortic SMCs. Whole-cell recordings from non-transfected (A) and A7r5 cells over-expressing AT1R (B–D). (A, B) IV relationships before ‘Basal', during hypotonic stimulation, ‘Hypo', and receptor stimulation with 1 μM vasopressin ‘VP' (left) and current density analyses at ±60 mV (right) are displayed. (C, D) Current time courses at ±60 mV with zero current levels (stippled lines); time scale bars 60 s and 100 pA (left) and current densities in the presence of neutralizing antibody, ‘nAb' (right) are displayed. (E) [Ca2+]i increases by hypotonic stimulation in HEK293 cells transfected with different amounts of AT1R-Venus cDNAs. Venus fluorescence was correlated with receptor densities. Numbers indicate the number of summarized cells and of independent transfections. (F) Concentration–response curves to AII of fura-2-loaded HEK293 cells stably expressing AT1R-Venus in isotonic and in hypotonic (273 mOsm kg−1) solutions.
To test whether AII potentially stored in A7r5 cells was released upon hypotonic cell swelling to activate AT1R in an autocrine manner, we blocked AII with a neutralizing antibody. Although a concentration of 100 μg ml−1 in the bath solution was sufficient to preclude the response to 100 nM exogenous AII (Figure 6C), the antibody had no effect on the cation current increase in response to osmotically induced membrane stretch (Figure 6D), clearly demonstrating that secreted AII is not involved in stretch-induced AT1R activation.
To investigate whether increasing AT1R densities correlate with cellular responses to membrane stretch, we monitored the increase in [Ca2+]i in response to hypoosmotic bath solutions in HEK293 cells transiently expressing AT1R at different receptor densities. Up to 2300 fmol AT1R per mg protein, there is a linear correlation between receptor density and the amplitude of the evoked Ca2+ transients (Figure 6E). When increasing AT1R density further, saturation was reached. Thus, AT1R density appears to be a critical variable determining whether ligand-independent receptor activation by membrane stretch is translated into detectable biological responses.
Under physiological conditions, vascular vasopressor receptors are simultaneously exposed to membrane stretch and agonists. As shown in Figure 6F, in HEK293 cells expressing recombinant AT1R, osmotically induced membrane stretch results in a leftward shift of the concentration–response curve to AII, thus sensitizing the response to agonist.
AT1R blockade reduces myogenic tone
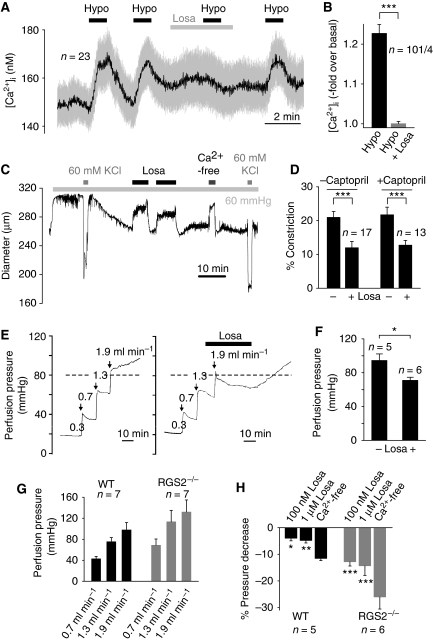
Next, we studied single isolated primary renal VSMCs with a high endogenous density of AT1Rs and measured the responses to osmotically induced membrane stretch. Fura-2-loaded VSMCs repeatedly responded to hypotonic bath solutions (250 mOsm kg−1) with transient rises of [Ca2+]i. The Ca2+ response was greatly diminished upon losartan application (Figure 7A and B), indicating that membrane stretch leads to AT1R activation in VSMCs.
Figure 7.
Physiological relevance of agonist-independent activation of AT1R by membrane stretch. (A) Representative [Ca2+]i recordings in fura-2-loaded renal VSMCs. Application of hypotonic stimuli, ‘Hypo', and losartan, ‘Losa', are indicated. (B) Summary of maximal [Ca2+]i increases in renal VSMCs. (C) Representative trace of a diameter recording from an isolated rat cerebral artery continuously superfused with oxygenated PSS. Application of 60 mmHg intravascular pressure, applications of 60 mM high K+ solution, application of nominally Ca2+-free solution and applications of 1 μM losartan are represented. (D) Summary of measured cerebral arteries showing the reduction of vessel constriction with losartan in the presence or absence of 1 μM captopril. (E) Representative traces of perfusion pressure recordings from isolated kidneys of mice in the absence and presence of losartan. (F) Summary of perfusion pressures. (G) Summary of perfusion pressures at indicated flow rates measured in perfused isolated kidneys from wild-type, ‘WT', and RGS2−/− mice. (H) Summary of flow pressure decrease after 5 min measured at a flow rate of 1.9 ml min−1 illustrating the pressure decrease with losartan compared with a Ca2+-free bath solution. Captopril had no effect on perfusion pressure.
To investigate whether the mechanosensitivity of AT1Rs has an important function for myogenic vasoconstriction, we monitored the effect of intravascular pressure on the diameter of isolated rat cerebral arteries in the presence and absence of the inverse AT1R agonist losartan. An intravascular pressure of 60 mmHg was applied to initiate myogenic vasoconstriction (Figure 7C). Maximal constriction of cerebral arteries was achieved by elevation of external K+ to 60 mM leading to activation of Cavs and a subsequent increase in intracellular Ca2+ concentration ([Ca2+]i). Addition of losartan diminished pressure-induced vasoconstriction, an effect that was reversed by washout of the drug. Maximal vasodilation was observed in a nominally Ca2+-free bath solution.
To investigate the possible contribution of AII locally released in the vessel wall, vessels were incubated with captopril to block the angiotensin-converting enzyme (ACE). In contrast to humans, only ACE is responsible for local AII formation in vascular tissues of rats (Jin et al, 2000). Figure 7D shows that myogenic constriction was markedly suppressed by losartan irrespective of previous captopril treatment. Accordingly, we did not obtain any evidence for a release of AII into the bath solution during the experiments (Supplementary data).
To monitor the myogenic response of a whole vascular bed, we performed perfusion experiments on isolated mouse kidneys (Figure 7E). The perfusion pressure monitored 10 min after applying an increase in flow to 1.9 ml min−1 was reduced by losartan infusion by 23.5 mmHg (P=0.013) (Figure 7F). Similar results were obtained in the presence of captopril (100 nM and 1 μM; n=5 each). The regulator of G protein signalling (RGS)-2 is a selective and negative regulator of Gq/11 proteins in the cardiovascular system (Xie and Palmer, 2007). Accordingly, RGS2−/− mice are characterized by an increased myogenic tone of isolated renal interlobar arteries (Hercule et al, 2007). In line with this observation, we observed an increased perfusion pressure at different flow rates in RGS2−/− kidneys compared with wild-type kidneys (Figure 7G). The increased perfusion pressure in RGS2−/− and wild-type kidneys was not affected by captopril (1 μM, at 0.7 and 1.9 ml min−1). Figure 7H shows that losartan had a more pronounced inhibitory effect on the myogenic response in RGS2−/− as compared with wild-type kidneys most likely reflecting the inhibition of a Gq/11-mediated signalling pathway by the inverse agonist. The pressure decrease by losartan was concentration dependent. Collectively, our findings are compatible with the notion that ligand-independent signalling of the AT1R is an essential component of dynamic mechanochemical signalling in VSM contributing to myogenic tone.
Discussion
Although TRPC channels have been implicated as essential elements mediating myogenic vasoconstriction, the mechanism by which pressure activates vascular TRPC channels is incompletely understood. TRPC channels have been shown to be activated by PLC (Clapham, 2003), possibly through direct actions of DAG (Hofmann et al, 1999; Inoue et al, 2001) generated through PLC activity. Pressure increases PLC activity (Osol et al, 1993) as well as the concentration of DAG (Narayanan et al, 1994) in isolated cerebral arteries. In this study, we provide evidence that Gq/11-coupled receptors are essential mechanosensing components in vascular smooth muscle cells (VSMCs) and function as sensors of membrane stretch leading to TRPC channel activation in a G protein- and PLC-dependent manner. Mechanically activated receptors adopt an active conformation allowing for productive G protein coupling and recruitment of β-arrestin. We therefore suggest that Gq/11-coupled receptors, for example AT1R, serve as mechanosensors mediating pressure-dependent, TRPC-mediated membrane depolarization in cerebral and renal arteries, which is at least in part responsible for myogenic vasoconstriction. Although our data indicate that AT1Rs have a paramount function in resistance arteries, we suggest that pressure activation of other Gq/11-coupled receptors, for example H1Rs and ETARs, may exhibit similar and synergistic effects in other myogenic arteries, thereby determining tissue-specificity of myogenic arteries.
Conceptually, the most straightforward way to explain the reported involvement of TRPC6 in the myogenic response (Welsh et al, 2002) would be to conjecture that TRPC6 is a stretch-activated cation channel mediating depolarization of VMSCs. In this vein, other TRP channels including TRPV2 (Muraki et al, 2003), TRPM4 (Earley et al, 2004), TRPM7 (Numata et al, 2007) and TRPC1 (Maroto et al, 2005) have also been implicated as potential molecular correlates of stretch-activated cation channels.
In isolated VSMCs from resistance arteries, a positive pipette pressure of 10 cm H2O in the whole-cell configuration is sufficient to evoke stretch-activated cation currents (Setoguchi et al, 1997). However, in isolated inside-out and cell-attached membrane patches from TRPC6-expressing HEK293 cells, such pressure values failed to elicit cation currents, although stretch-activated Cl− channels were promptly activated. Increasing the negative pipette pressure up to −70 cm H2O (corresponding to 50 mmHg) did not result in the rapid opening of cation channels after application of the suction pulse, thus contrasting with the typical behaviour of bona fide stretch-activated ion channels (Hamill, 2006). Moreover, our single channel recordings in the cell-attached configuration showed enhanced channel activity in response to a negative pipette pressure of 10 cm H2O only when TRPC6 channels were co-expressed with Gq/11-coupled receptors, but not when TRPC6 channels were expressed alone. These results provide compelling support for the notion that TRPC channels are mechanically activated through GPCRs.
Our conclusions are in line with a recent study (Gottlieb et al, 2008) but contrast with a report about the direct activation of recombinant TRPC6 by stretch in isolated inside-out membrane patches (Spassova et al, 2006). In the latter study, the threshold pressure level needed to detect TRPC6 activation was found to be higher than 80 mmHg (Spassova et al, 2006). Thus, it would appear that TRPC6 activation requires higher pressures than those needed for the large conductance Escherichia coli mechanosensitive ion channel, which displays pressure values for half-maximal activation of about 80 mmHg and functions as a last-resort safety valve (Levina et al, 1999). However, mechanosensitive channels in mammalian cells should be tuned to lower-pressure set points to be able to participate in the dynamic regulation of physiological processes.
It has been reported that in cardiomyocytes, mechanical stress can activate the AT1R independently of AII (Zou et al, 2004). In contrast to the latter report, the formyl peptide receptor in leukocytes analysed by a dynamic FRET technique was found to be inhibited by mechanical stress, thereby decreasing its constitutive activity and reducing pseudopod formation of leukocytes (Makino et al, 2006). Thus, it is an unresolved issue whether mechanical stress activates or inhibits GPCRs and whether such events are pertinent to physiological regulation.
Using a BRET approach, we show that membrane stretch allows the receptor to assume an active conformation, which is recognized by arrestins as avidly as that stabilized by agonist. We can only speculate about possible events leading to receptor activation by membrane stretch. A mechanically induced change of the lateral pressure profile exerted by the lipid bilayer of the plasma membrane (Kung, 2005) or mechanical activation of proteins directly connected to the receptor may result in a shift in the distribution between active and inactive conformations, which have recently been characterized depending on mechanical stretch in the case of the AT1R (Yasuda et al, 2008).
Taken together, these results impose a considerable revision of our present views of myogenic constriction (Bayliss effect), in particular, and G protein-coupled receptor function and regulation, in general. Our results suggest that Gq/11-coupled receptors function as sensors of membrane stretch in VSMCs and contribute to myogenic vasoconstriction of renal and cerebral arteries, emphasizing the novel concept that activation of TRPC channels by mechanical stimuli occurs through activation of GPCRs. GPCRs appear to be characterized by polymodal activation mechanisms and may serve as sensors for external chemical signals and transmembrane potential (Ben-Chaim et al, 2006). Recent data on orphan GPCRs (Levoye et al, 2006) and our findings suggest physiologically relevant, ligand-independent functions for GPCRs. Future investigations on ligand-independent signalling of GPCRs in other organs will be enlightening in this respect.
Materials and methods
Analysis of myogenic tone in cerebral arteries
After decapitation of anesthetized male Sprague–Dawley rats, brains were removed and transferred to cold (4°C), oxygenated (95% O2 and 5% CO2) physiological salt solution (PSS). Distal posterior cerebral and distal anterior cerebellar arteries were cannulated, allowing for application of intravascular pressure (for details, see Gollasch et al, 1998; Lohn et al, 2002).
Analysis of myogenic tone in isolated perfused kidneys
Isolated kidneys of male wild-type and RGS2−/− mice were perfused in an organ chamber using a peristaltic pump at constant flow (0.3–1.9 ml min−1) of oxygenated (95% O2 and 5% CO2) PSS. Perfusion pressure was determined by a pressure transducer after an equilibration period of 60–90 min. Data were recorded and analysed using a Powerlab acquisition system (Fesus et al, 2007).
Bioluminescence resonance energy transfer assay
To detect β-arrestin-2 recruitment by the human AT1R, the BRET1 technique was performed using a Fluostar Optima (BMG LabTechnologies, Offenburg, Germany) as described previously (Charest and Bouvier, 2003). Briefly, COS-7 cells were co-transfected with the cDNA encoding an AT1R-Venus fusion protein (10 μg) and 15 ng of a cDNA encoding a fusion protein between the humanized version of Renilla luciferase and rat β-arrestin-2 (βArr2-Rluc) using the FuGENE6 reagent. Both cDNAs were kindly provided by the laboratory of Dr Bouvier, Montréal. Forty-eight hours post-transfection, approximately 75 000 cells were seeded into a 96-well microplate in 100 μl of a bath solution containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose and 10 mM HEPES (pH 7.4), with an osmolality of 298–302 mOsm kg−1. Agonist-promoted arrestin recruitment was detected after adding 1 μM (final) AII. Hypo-osmotic stress was induced by adding 10 μl pure H2O (resulting in an osmolality of 273 mOsm kg−1). After 60 s, the osmotic stress was relieved from the cells by adding 10 μl of a two-fold concentrated bath solution. Effects of the AT1R antagonist losartan were determined by adding 1 μM (final) of the antagonist 4 min before agonist or hypoosmotic stimulation. The Rluc substrate coelenterazine H (5 μM) was added 25 min after stimulation and the BRET signal was continually sampled. The BRET signal (ratio of the light intensity measured at 535±30 over 475±30 nm) was determined in quadruplicates. BRET signals of cells expressing energy donor and acceptor were not significantly different (P=0.27) from cells expressing only the energy donor. The means of three independent experiments (monitored within 50 min) were calculated.
Electrophysiological techniques
HEK293 cells were transfected with cDNAs coding for human TRPC3, TRPC6 and mouse TRPC7 in pcDNA3, with the original cDNA coding for one of the following receptors: guinea pig histamine receptor 1 (H1R), rat type-5 muscarinic acetylcholine receptor (M5R), mouse angiotensin II AT1A (AT1R) and the enhanced green fluorescent protein (eGFP) reporter plasmid using the FuGENE6 reagent. A7r5 cells were transfected with the AT1R in pIRES2-EGFP (Clontech, Palo Alto, CA, USA). Conventional whole-cell patch-clamp recordings were carried out at 23°C at 48 h after transfection. The bath solution contained 110 mM NaCl, 5 mM CsCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose and 10 mM HEPES (pH 7.4 with NaOH) supplemented with mannitol to 300 mOsm kg−1. The hypoosmotic solution without mannitol had an osmolality of 249–253 mOsm kg−1. A7r5 cells were superfused with the isotonic bath solution plus 10 μM nicardipine and 50 μM NPPB. Data were collected with an EPC10 patch clamp amplifier (HEKA, Lambrecht, Germany) using the Pulse software. IV relations were obtained from triangular voltage ramps from −100 to +60 mV, with a slope of 0.4 V s−1 applied at a frequency of 1 Hz. Data were acquired at a frequency of 5 kHz after filtering at 1.67 kHz. Cell inflation was performed by applying a positive pressure of 8–10 cm H2O to the patch pipette through a water manometer. Membrane stretch was applied by raising the patch pipette vertically by 8–10 μm.
Single-channel recordings
HEK293 cells were transiently transfected with 2 μg of cDNAs coding for human TRPC6 in pIRES2-EGFP (Clontech, Palo Alto, CA, USA) or were cotransfected with TRPC6 and 0.5 μg cDNA coding for the H1R using 6 μl or 7.5 μl of the FuGENE6 reagent, respectively. For inside-out experiments, the cells were seeded on culture dishes treated with poly-L-lysine. Cell-attached and excised inside-out patch-clamp recordings were carried out at room temperature (23°C) 72 h after transfection. The bath and pipette solution for inside-out experiments contained 130 mM caesium methane-sulfonate, 102 μM CsCl, 1 mM MgCl2, 3.949 mM CaCl2, 10 mM BAPTA (100 nM free Ca2+) and 10 mM HEPES (pH 7.2 with CsOH), resulting in an osmolality of 294 mOsm kg−1. For cell-attached measurements, the same pipette solution was used and the bath solution contained 130 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES and 10 mM glucose (pH 7.4 with KOH), resulting in an osmolality of 294 mOsm kg−1 to define the membrane potential. The bath solutions also contained 50 μM NPPB. Patch pipettes made of borosilicate glass (Science Products, Hofheim, Germany) were coated with R-6101 (Dow Chemical, Midland, MI, USA) and had resistances of 8–10 MΩ. Data were collected with an EPC9 patch clamp amplifier (HEKA, Lambrecht, Germany) using the Pulse software and were acquired at a holding potential of −60 mV with a frequency of 20 kHz after filtering at 6.67 kHz. Membrane stretch during inside-out or cell-attached recordings was performed by applying negative pressure of 10 or 70 cm H2O to the patch pipette through a water manometer. Finally, in the inside-out configuration, 10 μM SAG was applied to the bath solution to obtain maximal channel activity in the patch. For the evaluation of consecutive channel activity (‘NPo', the product of the number of channels and open probability) in 1-s steps, PC DAC 1.1.5 of Marburg University Software Team was used.
Non-enzymatic isolation of renal VSMCs
Kidneys from 9- to 14-day-old mice were decapsulated and longitudinally dissected and the medullae were removed. Kidney halves were pressed through a series of stainless sieves with decreasing pore sizes (420 and 300 μm). Renal arteries were collected on a 100-μm sieve and transferred to collagen-coated coverslips and cultured with Dulbecco's modified Eagle medium and Ham's F12 (1:1) supplemented with 10% fetal calf serum and penicillin (100 U ml−1) and streptomycin (0.1 mg ml−1) for up to 6 days (37°C and 7% CO2).
Statistical analysis
Data are presented as means±standard error of the mean (s.e.m.). Unless stated otherwise, data were compared by a paired or unpaired Student's t-test, if a Gaussian distribution was confirmed by applying a Shapiro–Wilk (normality) test, and significance was accepted at P<0.05. For multiple comparisons, the one-way analysis of variance with Scheffé post hoc means comparison was applied. *P<0.05, **P<0.01, ***P<0.001, n.s. P⩾0.05.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Eva Braun, Fatma Colakoglu and Marga Losekam for technical assistance. We are grateful to Diana Herold and Yoland Anistan for expert assistance with myogenic tone experiments. We are indebted to Tim Plant for critical reading and providing A7r5 cells. We thank Kai Hoffmann (AstraZeneca) and Michela Kuffer (MSD) for a general supply of candesartan and losartan and Alexander Oksche for supplying darusentan. Thanks to Lutz Hein, Yasuo Mori, Torsten Schöneberg and Christof Zitt for AT1-, TRPC7-, GαΔ6qi4myr- and TRPC3-cDNA. This work was supported by Deutsche Forschungsgemeinschaft.
References
- Beech DJ, Muraki K, Flemming R (2004) Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol 559: 685–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H (2006) Movement of ‘gating charge' is coupled to ligand binding in a G-protein-coupled receptor. Nature 444: 106–109 [DOI] [PubMed] [Google Scholar]
- Charest PG, Bouvier M (2003) Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem 278: 41541–41551 [DOI] [PubMed] [Google Scholar]
- Clapham DE (2003) TRP channels as cellular sensors. Nature 426: 517–524 [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423 [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L (2005) Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25: 6980–6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Waldron BJ, Brayden JE (2004) Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929 [DOI] [PubMed] [Google Scholar]
- Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M (2007) Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75: 719–727 [DOI] [PubMed] [Google Scholar]
- Gollasch M, Wellman GC, Knot HJ, Jaggar JH, Damon DH, Bonev AD, Nelson MT (1998) Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res 83: 1104–1114 [DOI] [PubMed] [Google Scholar]
- Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E (2008) Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch 455: 1097–1103 [DOI] [PubMed] [Google Scholar]
- Hamill OP (2006) Twenty odd years of stretch-sensitive channels. Pflugers Arch 453: 333–351 [DOI] [PubMed] [Google Scholar]
- Hercule HC, Tank J, Plehm R, Wellner M, da Costa Goncalves AC, Gollasch M, Diedrich A, Jordan J, Luft FC, Gross V (2007) Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp Physiol 92: 1014–1022 [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263 [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99: 7461–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE (2006) Cellular mechanotransduction: putting all the pieces together again. FASEB J 20: 811–827 [DOI] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y (2006) Transient receptor potential channels in cardiovascular function and disease. Circ Res 99: 119–131 [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y (2001) The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res 88: 325–332 [DOI] [PubMed] [Google Scholar]
- Jin D, Takai S, Yamada M, Sakaguchi M, Miyazaki M (2000) The functional ratio of chymase and angiotensin converting enzyme in angiotensin I-induced vascular contraction in monkeys, dogs and rats. Jpn J Pharmacol 84: 449–454 [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD (2002) TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol 282: C347–C359 [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT (1995) Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol 269: H348–H355 [DOI] [PubMed] [Google Scholar]
- Kung C (2005) A possible unifying principle for mechanosensation. Nature 436: 647–654 [DOI] [PubMed] [Google Scholar]
- Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J 18: 1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Jockers R (2006) Do orphan G-protein-coupled receptors have ligand-independent functions? New insights from receptor heterodimers. EMBO Rep 7: 1094–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM (2002) Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063 [DOI] [PubMed] [Google Scholar]
- Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao W, Schmid-Schonbein GW (2006) G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol 290: C1633–C1639 [DOI] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP (2005) TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185 [DOI] [PubMed] [Google Scholar]
- Martinac B (2004) Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci 117: 2449–2460 [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V (2002) The TRP channels, a remarkably functional family. Cell 108: 595–598 [DOI] [PubMed] [Google Scholar]
- Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N (2003) Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 22: 6027–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838 [DOI] [PubMed] [Google Scholar]
- Murphy TV, Spurrell BE, Hill MA (2002) Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways. Clin Exp Pharmacol Physiol 29: 612–619 [DOI] [PubMed] [Google Scholar]
- Narayanan J, Imig M, Roman RJ, Harder DR (1994) Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol 266: H1840–H1845 [DOI] [PubMed] [Google Scholar]
- Nickenig G, Strehlow K, Roeling J, Zolk O, Knorr A, Bohm M (1998) Salt induces vascular AT1 receptor overexpression in vitro and in vivo. Hypertension 31: 1272–1277 [DOI] [PubMed] [Google Scholar]
- Numata T, Shimizu T, Okada Y (2007) TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol 292: C460–C467 [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA (2006) Mechanisms of mechanotransduction. Dev Cell 10: 11–20 [DOI] [PubMed] [Google Scholar]
- Osol G, Laher I, Kelley M (1993) Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol 265: H415–H420 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X, Wagner C, Chatziantoniou C, Kurtz A, Arendshorst WJ (1997) Regulation of angiotensin II receptor AT1 subtypes in renal afferent arterioles during chronic changes in sodium diet. J Clin Invest 99: 1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi M, Ohya Y, Abe I, Fujishima M (1997) Stretch-activated whole-cell currents in smooth muscle cells from mesenteric resistance artery of guinea-pig. J Physiol 501 (Part 2): 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL (2006) A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Nelson MT (2005) Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol 83: 215–242 [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE (2002) Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250 [DOI] [PubMed] [Google Scholar]
- Xie GX, Palmer PP (2007) How regulators of G protein signaling achieve selective regulation. J Mol Biol 366: 349–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I (2008) Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep 9: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I (2004) Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information