Abstract
The prevalence of metabolic syndrome is increasing along with breast cancer incidence worldwide. Since fenretinide improves insulin action and glucose tolerance in insulin-resistant obese mice and because tamoxifen has shown to regulate several markers involved in metabolic syndrome, we sought to investigate the effect of fenretinide or tamoxifen at low-dose on features linked to insulin resistance in premenopausal women at-risk for breast cancer.
We randomized 235 women to low-dose tamoxifen (5 mg/daily), or fenretinide (200 mg/daily), or their combination or placebo for two years. We employed the homeostasis model assessment (HOMA; fasting insulin*glucose/22.5) to estimate insulin sensitivity. Women were considered to improve insulin sensitivity when they shifted from a HOMA ≥2.8 to <2.8.
There was no effect of fenretinide or tamoxifen on HOMA overall, but overweight women (body mass index ≥25 kg/m2) had a 7-fold greater probability to normalize HOMA after two years of fenretinide treatment (OR=7.0; 95%CI: 1.2-40.5), with 25% of women improving their insulin sensitivity, whereas tamoxifen decreased insulin sensitivity by almost 7 times as compared to subjects not taking tamoxifen (OR=0.15; 95%CI: 0.03-0.88). In this group only 5% improved their insulin sensitivity. Interestingly, women with intraepithelial or microinvasive neoplasia had higher HOMA (3.0) than unaffected subjects (2.8; P=0.07).
Fenretinide can positively balance the metabolic profile in overweight premenopausal women and this may favorably affect breast cancer risk. Furthermore, features of the metabolic syndrome should be taken into consideration before proposing tamoxifen for breast cancer prevention. The clinical implications of these results require further investigations.
Keywords: risk biomarkers, insulin resistance, breast cancer prevention, HOMA
INTRODUCTION
Tamoxifen is FDA-approved for breast cancer risk reduction, but its use is associated with serious adverse effects (1). We have conducted several trials using lower doses of tamoxifen in an attempt to increase its therapeutic index (2, 3). Fenretinide is a synthetic vitamin A derivative that selectively accumulates in the breast tissue (4), exhibits apoptotic and anti-invasive properties in vitro and in vivo with a good toxicity profile (5, 6), that has shown a lower risk of second breast cancer in premenopausal women which persists several years after treatment cessation (7).
Because the combination of tamoxifen and fenretinide is synergistic in rodent mammary tumor models and its safety has already been demonstrated in clinical trials (8), we conducted a 2×2 chemoprevention trial in premenopausal women using the change in plasma IGF-I and mammographic density as surrogate-endpoint biomarkers. Preliminary results (3) showed that the combination of low-dose tamoxifen and fenretinide is safe, that tamoxifen reduces IGF-I levels by 15%, and that no synergistic effect between the two drugs was observed on the primary study endpoints.
The prevalence of metabolic syndrome and obesity is rapidly increasing in the Western world and evidence points to a link with breast cancer incidence (9, 10). Strategies are being developed to trim down breast cancer incidence and recurrence through insulin lowering drugs and dietary intervention combined with aerobic exercise programs to reduce insulin-resistance. Interestingly, Yang and colleagues (11) showed an improvement in insulin action and glucose tolerance by fenretinide in insulin-resistant obese mice, a finding which prompted us to investigate the ability of fenretinide to improve insulin sensitivity in humans. We were also interested in assessing the effect of tamoxifen at a lower dose on the HOMA index since previous studies were somewhat contradictory, tamoxifen being associated with a favorable profile of some lipids (2, 12, 13), but an increased risk of hypertriglyceridemia (14).
Within our trial, we employed the homeostasis model assessment (HOMA) as a surrogate index of insulin resistance (15). Women were randomized to receive either low-dose tamoxifen (5 mg/daily), or fenretinide (200 mg/daily), or their combination or placebo for two years. We considered women to improve insulin sensitivity when they switched from a HOMA status ≥ 2.8 to a HOMA < 2.8. This cut-off value corresponds to the lower limit of the highest quintile in a population-based study conducted in 888 subjects randomly selected from the general population in Bruneck, Italy (16).
PATIENTS AND METHODS
Eligibility criteria
Eligible subjects were premenopausal women with either an in situ breast cancer (n=160) or a small invasive breast cancer of favorable prognosis (pT1mic or pT1a, n=21) in the previous three years. Unaffected women (n=54) were eligible if they had a Gail 5-year risk for breast cancer >1.7%. The main subject characteristics and the preliminary data have been published elsewhere (3). All subjects signed a consent form approved by the local Institutional Review Board.
Study Design
A 2×2 factorial design was adopted for this randomized double-blind placebo-controlled trial. Subjects were randomized to two years treatment of either low-dose tamoxifen (5 mg/daily), or fenretinide (200 mg/daily), or their combination, or placebo for two years. A monthly 3-day interruption of fenretinide was introduced to allow the partial recovery of retinal storage. Six-month follow-up continued after treatment completion for at least 5 years. There were two randomization strata, high risk women identified from the Gail Model and affected women with in situ or pT1mic/pT1a breast cancer. The study was conducted in two centers: the European Institute of Oncology, Milan, where 91% of subjects were recruited, and the Division of Medical Oncology, Vicenza, Italy.
Assay methods
Fasting blood samples for circulating biomarkers were collected and stored at -80°C until centrally assayed. Serum glucose concentration and lipid profile were determined on fresh samples by enzymatic method with a Cobas Integra 800 (Roche Diagnostics S.p.A.). Circulating serum insulin levels were measured by radioimmunoassay (RIA) kits purchased from Diagnostic System Laboratories Inc. (Webster, TX, USA). The sensitivity of the test was 1.3 μIU/mL, while intra- and inter-assay CV of our in-house pooled serum control sample (mean 14.9 IU/mL) were 11.5 % and 15.2%, respectively. Plasma IGF-I was determined on EDTA by chemiluminescent immunometric assay method (Nichols Institute Diagnostics, San Juan, CA). The assay was performed on the automatic instrument LIAISON (Diasorin SpA, Saluggia, Italy). The sensitivity of the test was 0.8 nmol/L, whereas the intra- and interassay CV of our in-house pooled serum control sample were 5.4% and 8.2%, respectively. Total, high-density lipoprotein, low-density lipoprotein cholesterol, and triglycerides were measured by enzymatic luminescent methods with COBAS INTEGRA 800 (Roche Diagnostics). Plasma leptin concentrations were measured using a RIA kit for human leptin (Linco Research Inc., St. Louis, MO, USA). In this assay, the detection limit is 0.5 ng/mL; the intra-assay CV is 2.2% (5.9 ng/mL), 2.7% (25 ng/mL) and 5.9% (62.6 ng/mL); the inter-assay precision from 10 different runs of 3 patients serum samples was 4.3%, 4%, 6.9% at the concentration of 5.1, 20.9 and 56.1 ng/mL, respectively. Plasma retinol levels were measured by HPLC using the method previously described (17).
We employed the homeostasis model assessment (HOMA) as a surrogate index of insulin sensitivity, i.e., [fasting insulinemia (mU/L) x glycemia (mmol/L)]/22.5 (15).
Statistical analysis
Descriptive statistics were first used to characterize the study population. The analysis was carried out for the whole population and separately for overweight/obese women (BMI≥25) and normal weight subjects (BMI<25). Spearman correlation coefficients (r) were calculated to study the relationships between circulating biomarkers and BMI. GLM models were also applied for the evaluation at baseline of the association between leptin and HOMA index, adjusting for BMI and randomization strata. Odds ratios were calculated to assess the probability of having an in situ or a pT1mic/pT1a breast cancer as compared to unaffected high risk women by unit of increase in HOMA index.
We evaluated the frequencies of subjects with HOMA≥2.8 at baseline and HOMA<2.8 at the first, second and third year. Women were considered to improve insulin sensitivity when they passed from HOMA≥2.8 to HOMA<2.8, using the cut-off value previously described (16). Frequency differences by treatment groups were assessed using Chi-square tests for independence of categorical variables and risk analyses were done using logistic regression. Odds ratios assessed the probability of improving insulin sensitivity status by fenretinide and tamoxifen treatment through logistic regression adjusting for treatment allocation.
Values of IGF-I, total cholesterol, HDL and LDL cholesterol and triglycerides were analyzed through a repeated-measure ANCOVA model at I and II year of treatment. Since there was no statistically significant interaction between drugs, we compared the women who had taken fenretinide with those who did not, irrespective of the combination treatment (placebo or tamoxifen), and women on tamoxifen with those who were not, irrespective of the combination treatment (placebo or fenretinide). This allowed us to gain statistical power. Mixed effects models were adjusted for baseline value and included as fixed effects: time, treatment (tamoxifen and fenretinide as indicators variables), HOMA index and interactions of treatment with HOMA index. Use of mixed-effect models and the Kenward-Roger technique for determining denominator degrees of freedom were adopted (PROC MIXED, SAS) (18). Log transformations were used when necessary to achieve normality. Predicted values of the biomarkers were plotted against HOMA index when we found a significant interaction of treatment by HOMA index.
Data were analyzed using the SAS System Software for Windows, release 8.0. (SAS Institute, Cary, NC, USA) (18)
RESULTS
Table 1 shows descriptive statistics of the study population according to treatment allocation. In total, 171 (73%) women had a normal BMI, 47 (20%) were overweight (BMI≥25 kg/m2) and 16 (7%) obese (BMI≥30 kg/m2). Of the women with a normal BMI, 49% had a HOMA index≥2.8, while those with a BMI≥25 showed a higher percentage of HOMA index≥2.8 (59%). The frequencies of women at low and high insulin sensitivity were distributed evenly between treatment arms (P=0.48). Likewise, there were no statistically significant differences between groups as regards circulating biomarkers linked to insulin resistance and retinol levels. Furthermore, we assessed the correlation between biomarkers (data not shown). HOMA index (r=0.357), leptin (r=0.688), HDL cholesterol (r=-0.266), and triglycerides (r=0.228) were all significantly associated with BMI at baseline (P<0.001). Predictors of HOMA index were glycemia (r=0.573; P<0.001), BMI (r=0.357; P<0.001), leptin (r=0.320; P<0.001), and retinol (r=0.177; P<0.01).
Table 1.
Baseline characteristics (frequencies and mean ± SD) and biomarker levels (median and interquartile range) of participants according to treatment allocation
| Characteristics | Tamoxifen+Fenretinide | Tamoxifen+Placebo | Fenretinide+Placebo | Placebo+Placebo |
|---|---|---|---|---|
| Number of participants | 60 | 58 | 59 | 58 |
| Disease status Gail/LCIS/DCIS/T1 | 14/9/30/7 | 14/7/32/5 | 13/9/32/5 | 13/4/37/4 |
| Age at entry (yrs) | 46.9 ± 4.5 | 46.2 ± 5.0 | 46.2 ± 5.2 | 46.5 ± 4.3 |
| Body mass index (kg/m2) | 24.1 ± 3.8 | 24.1 ± 3.8 | 23.1 ± 3.4 | 23.1 ± 2.9 |
| Waist to hip girth ratio | 0.827 ± 0.066 | 0.816 ± 0.075 | 0.839 ± 0.074 | 0.819 ± 0.066 |
| HOMA Index | 2.8 (2.4-3.89) | 3.0 (2.2-3.9) | 3.2 (2.6-4.5) | 2.5 (1.9-3.6) |
| Glucose (mg/dL) | 88 (84-94) | 89 (83-95) | 89 (83-95) | 85 (80-91) |
| Insulin (mU/mL) | 12.9 (10.6-16.6) | 12.9 (10.6-16.9) | 14.9 (11.6-18.6) | 12.4 (9.5-16.3) |
| IGF-I (ng/mL) | 140 (118-182) | 130 (111-165) | 143 (116-182) | 146 (114-181) |
| Total cholesterol (mg/dL) | 211 (194-229) | 216 (184-236) | 208 (187-247) | 206 (183-227) |
| HDL cholesterol (mg/dL) | 69 (60-79) | 65 (57-78) | 71 (57-81) | 68 (60-78) |
| LDL cholesterol (mg/dL | 128 (106-145) | 121 (106-153) | 122 (103-151) | 123 (104-145) |
| Triglycerides (mg/dL) | 79 (60-93) | 79 (59-105) | 69 (53-100) | 65 (55-86) |
| Leptin (ng/mL) | 10.5 (6.9-14.1) | 10.9 (8.2-15.6) | 10.1 (6.3-15.6) | 9.8 (6.3-16.5) |
| Retinol (ng/mL) | 544 (458-635) | 548 (490-633) | 544 (476-657) | 540 (452-614) |
Gail: unaffected women with a 5-year risk for breast cancer ≥1.7%, according to the Gail breast cancer risk assessment model
LCIS: lobular carcinoma in situ
DCIS: ductal carcinoma in situ
T1: T1mic: <1mm invasion;and T1a: <5mm)
Table 2 shows the biomarker levels after 2 years of treatment. Based on a previous analysis showing no interaction between tamoxifen and fenretinide (3), the effect of either agent was assessed separately (fenretinide versus no fenretinide and tamoxifen versus no tamoxifen). IGF-I levels were reduced by tamoxifen treatment (P=0.01) and retinol by fenretinide (P=0.001). As retinol levels correlated with HOMA index, we checked for a possible interaction of HOMA index with retinol decline after fenretinide treatment, but this was not significant.
Table 2.
Median and interquartile ranges of biomarkers after two years treatment
| Biomarkers | Tamoxifen+Fenretinide | Tamoxifen+Placebo | Fenretinide+Placebo | Placebo+Placebo |
|---|---|---|---|---|
| HOMA Index | 2.9 (2.4-3.8) | 3.0 (2.3-3.5) | 2.6 (2.2-3.4) | 2.5 (1.9-3.5) |
| Glucose (mg/dL) | 86 (83-93) | 87 (81-93) | 89 (80-93) | 86 (82-93) |
| Insulin (mU/mL) | 13.0 (11.1-16.4) | 14.2 (10.6-16.1) | 12.2 (10.3-15.4) | 12.1 (8.9-15.3) |
| IGF-I (ng/mL) | 121 (88-158 ) | 118 (90-154) | 141 (122-166) | 140 (112-167) |
| Total cholesterol (mg/dL) | 198 (187-225) | 200 (186-235) | 214 (194-242) | 208 (188-230) |
| HDL cholesterol (mg/dL) | 71 (57-81) | 63 (52-78) | 74 (64-82) | 66 (58-73) |
| LDL cholesterol (mg/dL) | 112 (95-135) | 117 (100-134) | 126 (107-151) | 126 (105-151) |
| Triglycerides (mg/dL) | 69 (54-103) | 77 (66-111) | 64 (53-82) | 65 (52-90) |
| Leptin (ng/mL) | 11.3 (7.2-15.2) | 11.7 (8.4-16.5) | 10.0 (6.5-14.0) | 12.0 (7.0-15.3) |
| Retinol (ng/mL) | 194 (117-495) | 524 (484-608) | 206 (120-491) | 533 (444-610) |
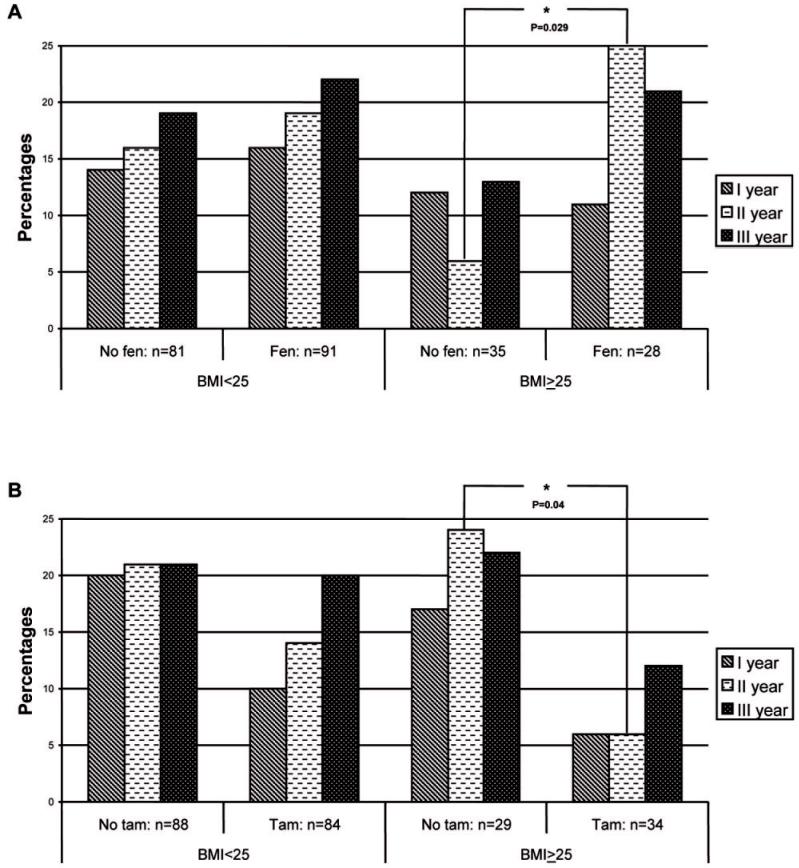
Figure 1 illustrates the changes over time in insulin sensitivity status by drug stratified by BMI. The findings, expressed as the percentage of women who shifted from HOMA≥2.8 to HOMA<2.8, show that overweight women (BMI≥25) taking fenretinide for 2 years had a 7-fold greater probability to improve insulin sensitivity than women not taking fenretinide (OR=7.0; 95%CI: 1.2-40.5; P=0.029, Figure 1, panel A). In contrast, tamoxifen decreased insulin sensitivity by nearly 7 times in overweight women (OR=0.15; 95%CI: 0.03-0.88; P=0.04, Figure 1, panel B). Neither agent showed any effect on insulin sensitivity in subjects with normal BMI. We also looked at the effect in each of the 4 allocated arms, and the insulin sentitizing effect of fenretinide was particularly evident in the “fenretinide+placebo” arm, where the median HOMA levels decreased from 5.2 to 2.5 after two years of treatment in this group.
Figure 1.
Effect of fenretinide (top panel, A; FEN: fenretinide+placebo or fenretinide+tamoxifen) and tamoxifen (top panel B; TAM: tamoxifen+placebo and tamoxifen+fenretinide) and homeostasis model assessment (HOMA) index. The histograms indicate the percentage of subjects improving their HOMA index from baseline, shifting from HOMA ≥ 2.8 to HOMA < 2.8. Two years treatment followed by one year follow-up.
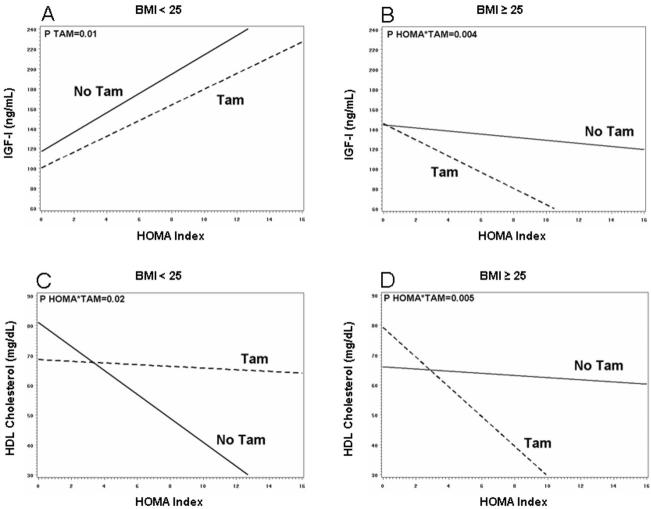
Figure 2 shows the differential effects of tamoxifen according to BMI category as regards the relationship between HOMA index and IGF-I (panel A and B) or HDL cholesterol (panel C and D). In women with normal BMI, IGF-I levels increased with increasing HOMA index and were significantly lowered by tamoxifen regardless of HOMA index values (P=0.01, fig 2, panel A). At variance, in overweight women the decrease exerted by tamoxifen was much greater with increasing HOMA index (P=0.004 for the HOMA*tamoxifen interaction, fig 2, panel B). In normal weight women, tamoxifen improved HDL-C as HOMA increased (P=0.02 for the HOMA*tamoxifen interaction, fig 2, panel C), whereas in overweight women tamoxifen decreased HDL-C as HOMA increased (P=0.001 for the HOMA*tamoxifen interaction, fig 2 panel D).
Figure 2.
Relationship between HOMA index and IGF-I (panel A and B) or HDL cholesterol (panel C and D) by BMI category according to treatment (TAM: tamoxifen+placebo or tamoxifen+fenretinide; FEN: fenretinide+placebo or fenretinide+tamoxifen). Lines represents the fitted values of the interaction between HOMA index and treatment, obtained from repeated-measure analysis of year I and II, after adjusting for baseline value, time, HOMA index and treatment. Women allocated to tamoxifen were compared with those who were not, irrespectively of combination treatment (placebo or fenretinide).
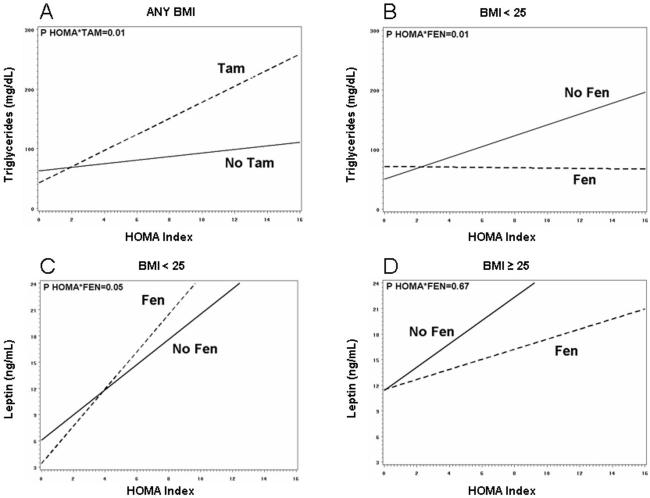
Figure 3 shows the different effects of the drugs according to BMI category on the relationship between HOMA index and triglycerides (panel A and B) or leptin (panel C and D). Tamoxifen increased triglyceride levels as HOMA increased irrespective of BMI (P=0.01 for the HOMA*tamoxifen interaction, fig 3, panel A). In contrast, in normal weight women fenretinide blunted the increase in triglycerides associated with the HOMA increase (P=0.01 for the HOMA*fenretinide interaction, fig 3, panel B). Finally, fenretinide had a different effect on leptin levels according to BMI, with a slight increase in leptin as HOMA increased in normal weight women (P=0.05 for the HOMA*fenretinide interaction, fig 3, panel C), as opposed to a non-significant decrease in overweight women.
Figure 3.
Relationship between HOMA index and triglycerides (panel A and B) or leptin (panel C and D) by BMI category according to treatment (TAM: tamoxifen+placebo or tamoxifen+fenretinide; FEN: fenretinide+placebo or fenretinide+tamoxifen) drugs as regards the. Lines for triglycerides represent the fitted values of the interactions between treatment and HOMA index, obtained from repeated-measure analysis considering year I and II, after adjusting for baseline value, time and treatment
Table 3 shows the median and interquartile ranges of HOMA index according to disease status. The median HOMA index value of unaffected high risk women overlapped the HOMA index reference value of 2.8, while women with intraepithelial or microinvasive breast cancer had a median HOMA index equal to 3.0. This translated into a 24% increased risk of bearing an intraepithelial or microinvasive breast cancer by unit increase in HOMA index (P=0.07).
Table 3.
Association between HOMA index and disease status. Median and interquartile ranges of HOMA index, including odds ratio estimates by HOMA index for having an in situ or a small invasive breast cancer as compared to unaffected high risk women
| Randomization strata | Median | Lowest quartile | Highest quartile |
|---|---|---|---|
| Unaffected high risk women (n=54) (5 year Gail risk ≥ 1.7%) | 2.77 | 1.82 | 3.62 |
| Intraepithelial or microinvasive breast cancer (n=181) | 2.99 | 2.24 | 4.00 |
| Odds ratio estimate | 95% Confidence Interval | ||
|---|---|---|---|
| Risk increase by unit of increase in HOMA index | 1.24 | 0.98-1.56 | |
DISCUSSION
The metabolic syndrome, which is characterized by visceral obesity, glucose intolerance, hyperinsulinemia, hypertension and dyslipidemia, has become a worldwide problem, and the mechanisms by which it promotes breast cancer development have recently been elucidated (19). Increased levels of insulin and insulin-like growth factor I have been causally linked to breast cancer (20, 21), with hyperinsulinemia which in turn amplifies the bioavailability of IGF-I (22). Insulin resistance develops as a metabolic adaptation to increased levels of circulating non-esterified fatty acids released from intra-abdominal adipose tissue that forces liver, muscles and other tissues to shift towards storage and oxidation of fats (9).
In our study we found that overweight and obese women taking fenretinide improved their HOMA-based insulin sensitivity score. This benefit was associated with a decrease in plasma leptin concentrations in overweight women as HOMA index increased. Moreover, fenretinide prevented triglyceride increase associated with HOMA increase in normal weight women. Leptin is mainly produced by the adipocytes, conveys information to the hypothalamus on the amount of energy stored in fat, and suppresses appetite. It is a mitogen for various cell types, including normal and transformed breast epithelial cells (23, 24). We found a highly positive correlation between leptin levels and BMI in our cohort of premenopausal women. The relationships between obesity, leptin, insulin-resistance and mammary tumor development are not fully understood. Genetically obese, leptin-deficient mice or mice lacking a functional leptin receptor (OB-R) fail to develop oncogene-induced mammary tumors (25, 26), but a direct association between high circulating leptin levels and breast cancer risk has not unequivocally been confirmed (27).
Interestingly, the insulin-sensitizing effect of fenretinide was observed only after the 2nd year of treatment, with a seven-fold greater probability to improve insulin sensitivity compared with women not taking fenretinide. We have no clear explanation for this time lag, which suggests a gradual activity of the drug on reversal of insulin-resistance and its persistence after treatment cessation. Fenretinide and N-(4-methoxyphenyl)retinamide, the most abundant metabolite in human plasma, are lipophilic and therefore accumulates in the adipose tissue were they are retained for several months after drug cessation (17). Fenretinide may enhance nuclear receptor heterodimerization promoting the synthesis of lipids and carbohydrates possibly favoring insulin-sensitivity (28).
A putative link between fenretinide and insulin-sensitivity may be ascribed to the likely reduction in retinol-binding protein 4 (RBP4) levels following the decrease in retinol levels. RBP4 is an adipocyte-secreted molecule that vehicles retinol in the blood through a specific binding site and is associated with several features of the metabolic syndrome (29). Improvement in insulin action in insulin-resistant subjects after exercise training is associated with a drop in serum RBP4 (30). Plasma concentrations of RBP4 decrease proportionally with retinol (31) and their decrease is highly correlated (r=0.96) during fenretinide administration (32). In our study, plasma retinol levels underwent a 50% decrease at 1st and 2nd year of treatment, then almost virtually recovered back to baseline values at 3rd year. While we were unable to measure RBP4 levels, we considered the retinol decrease as a surrogate marker of serum RBP4 drop. Serum levels of RBP4 are increased in insulin-resistant states (11, 30, 33), although the correlation is attenuated with increasing age (34), and experiments in mice suggest that elevated RBP4 levels cause insulin resistance by reducing IRS-1 tyrosine phosphorylation (11).
In contrast to fenretinide, low-dose tamoxifen was associated with increased HOMA index in overweight women. Moreover, tamoxifen worsened circulating levels of HDL-cholesterol and triglycerides in overweight women as HOMA index increased. Although several reports suggest that the predominant effects of tamoxifen on lipids are favorable, especially on LDL cholesterol (2, 12, 13), hypertriglyceridemia during tamoxifen 20 mg/day has been observed mostly in patients with family history of dyslipidemia and high pre-treatment triglyceride levels (35-37). Tamoxifen has also been associated with an increased risk of developing non-alcoholic steatohepatitis in overweight-obese women in association with abnormal elevations of alanine aminotransferase (38). In our study, we found that low-dose tamoxifen exerted a greater increase of triglyceride levels with increasing HOMA index, while there was no association between triglycerides and HOMA index in women not taking tamoxifen. Tamoxifen is known to reduce the hepatic production in IGF-I (2, 3). The drug also interacts with the IGF-I receptor signaling pathway, downregulating the down-stream cascade in breast cancer cells, including IRS-1 tyrosine phosphorylation (39, 40). One of the primary defects underlying insulin resistance is an impairment in post-receptor pathways of insulin action, bringing to a down-regulation of insulin receptor substance-1 (IRS-1) signaling by excess free fatty acids (41). In addition, the active metabolite 4-OH-tamoxifen may induce a marked inhibitory action on pancreatic beta-cell function in a rat model (42). Given the information above, we suggest that tamoxifen may increase insulin requirement in women carrying features of the metabolic syndrome. While circulating SHBG and IGFBP-1 rise with tamoxifen treatment (2), a phenomenon expected to positively influence insulin-sensitivity, our data suggest that in overweight women the insulin resistant effect of tamoxifen is predominant.
We observed a border-line significant association between HOMA index at baseline and disease status, which translated into a 24% increased risk of having intraepithelial or microinvasive breast cancer by every unit of increase in HOMA index relative to unaffected high-risk women (P=0.07). This finding is in line with the observation that postmenopausal breast cancer patients with metabolic syndrome or type 2 diabetes have an increased risk of recurrence (43, 44), and that premenopausal women with features of the metabolic syndrome (hyperandrogenism and luteal insufficiency) have an increased breast cancer risk (45-47). As the prevalence of metabolic syndrome is increasing steadily in the developing countries, intervention aimed at preventing insulin-resistance and obesity represent important health issues also for breast cancer prevention.
In conclusion, our results suggests that fenretinide positively balance the metabolic profile in obese women, offering some clues towards its evaluation in the treatment of metabolic syndrome. In contrast, tamoxifen, even at a lower dose, worsened insulin-sensitivity in obese subjects, providing further support to its careful use as a preventive agent in these women, where most serious adverse events tend to occur, including endometrial cancer (48, 49) and deep-vein thrombosis (50).
ACKNOWLEDGEMENTS
We thank Loredana Quadro for her technical expertise in the preparation of the manuscript. Fenretinide was manufactured and gifted by the RW Johnson Pharmaceutical Research Institute, Spring House, PA, USA. Tamoxifen was gifted by Laboratori MAG, Garbagnate, and manufactured by Cosmo SpA, Lainate, Italy.
The trial was supported by NCI grant number CA-77188, a contract from the Italian Foundation for Cancer Research (FIRC) and a regional grant (1068/2005) on second tumors from the Associazione Italiana per la Ricerca sul Cancro (AIRC).
REFERENCES
- 1.Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–46. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 2.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–90. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 3.Guerrieri-Gonzaga A, Robertson C, Bonanni B, et al. Preliminary results on safety and activity of a randomized, double-blind, 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in premenopausal women. J Clin Oncol. 2006;24:129–35. doi: 10.1200/JCO.2005.02.9934. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RG, Moon RC, Hawthorne M, Formelli F, Costa A. Distribution of fenretinide in the mammary gland of breast cancer patients. Eur J Cancer. 1991;27:138–41. doi: 10.1016/0277-5379(91)90471-o. [DOI] [PubMed] [Google Scholar]
- 5.Costa A, Malone W, Perloff M, et al. Tolerability of the synthetic retinoid Fenretinide (HPR) Eur J Cancer Clin Oncol. 1989;25:805–8. doi: 10.1016/0277-5379(89)90124-7. [DOI] [PubMed] [Google Scholar]
- 6.Rotmensz N, De Palo G, Formelli F, et al. Long-term tolerability of fenretinide (4-HPR) in breast cancer patients. Eur J Cancer. 1991;27:1127–31. doi: 10.1016/0277-5379(91)90309-2. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Mariani L, Decensi A, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17:1065–71. doi: 10.1093/annonc/mdl047. [DOI] [PubMed] [Google Scholar]
- 8.Conley B, O’shaughnessy J, Prindiville S, et al. Pilot trial of the safety, tolerability, and retinoid levels of N-(4-hydroxyphenyl) retinamide in combination with tamoxifen in patients at high risk for developing invasive breast cancer. J Clin Oncol. 2000;18:275–83. doi: 10.1200/JCO.2000.18.2.275. [DOI] [PubMed] [Google Scholar]
- 9.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–92. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 10.Stoll BA. Western nutrition and the insulin resistance syndrome: a link to breast cancer. Eur J Clin Nutr. 1999;53:83–7. doi: 10.1038/sj.ejcn.1600700. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 12.Grey AB, Stapleton JP, Evans MC, Reid IR. The effect of the anti-estrogen tamoxifen on cardiovascular risk factors in normal postmenopausal women. J Clin Endocrinol Metab. 1995;80:3191–5. doi: 10.1210/jcem.80.11.7593425. [DOI] [PubMed] [Google Scholar]
- 13.Bagdade JD, Wolter J, Subbaiah PV, Ryan W. Effects of tamoxifen treatment on plasma lipids and lipoprotein lipid composition. J Clin Endocrinol Metab. 1990;70:1132–5. doi: 10.1210/jcem-70-4-1132. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, Maisonneuve P, Rotmensz N, et al. Tamoxifen for the Prevention of Breast Cancer: Late Results of the Italian Randomized Tamoxifen Prevention Trial Among Women With Hysterectomy. JNCI Journal of the National Cancer Institute. 2007;99:727–37. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643–9. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 17.Formelli F, Clerici M, Campa T, et al. Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol. 1993;11:2036–42. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- 18.SAS Institute Inc. SAS/STATTM User’s Guide. SAS Institute Inc.. SAS Institute; USA: Cary, NC: 1990. [Google Scholar]
- 19.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin PJ, Ennis M, Bahl M, et al. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 21.Bruning PF, Bonfrer JM, Van Noord PA, Hart AA, de Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer. 1992;52:511–6. doi: 10.1002/ijc.2910520402. [DOI] [PubMed] [Google Scholar]
- 22.Fair AM, Dai Q, Shu XO, et al. Energy balance, insulin resistance biomarkers, and breast cancer risk. Cancer Detect Prev. 2007;31:214–9. doi: 10.1016/j.cdp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–11. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 24.Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50–5. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 25.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–93. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 26.Cleary MP, Phillips FC, Getzin SC, et al. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 27.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 28.Metzger D, Imai T, Jiang M, et al. Functional role of RXRs and PPARgamma in mature adipocytes. Prostaglandins Leukot Essent Fatty Acids. 2005;73:51–8. doi: 10.1016/j.plefa.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 2007;50:814–23. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]
- 30.Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 31.Gamble MV, Ramakrishnan R, Palafox NA, Briand K, Berglund L, Blaner WS. Retinol binding protein as a surrogate measure for serum retinol: studies in vitamin A-deficient children from the Republic of the Marshall Islands. Am J Clin Nutr. 2001;73:594–601. doi: 10.1093/ajcn/73.3.594. [DOI] [PubMed] [Google Scholar]
- 32.Formelli F, Carsana R, Costa A, et al. Plasma retinol level reduction by the synthetic retinoid fenretinide: a one year follow-up study of breast cancer patients. Cancer Res. 1989;49:6149–52. [PubMed] [Google Scholar]
- 33.Qi Q, Yu Z, Ye X, et al. Elevated Retinol-Binding Protein 4 Levels are Associated with Metabolic Syndrome in Chinese People. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 34.Gavi S, Qurashi S, Stuart LM, et al. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity (Silver Spring) 2008;16:893–5. doi: 10.1038/oby.2007.138. [DOI] [PubMed] [Google Scholar]
- 35.Elisaf MS, Nakou K, Liamis G, Pavlidis NA. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067–9. doi: 10.1023/a:1008309613082. [DOI] [PubMed] [Google Scholar]
- 36.Hozumi Y, Kawano M, Miyata M. Severe hypertriglyceridemia caused by tamoxifen-treatment after breast cancer surgery. Endocr J. 1997;44:745–9. doi: 10.1507/endocrj.44.745. [DOI] [PubMed] [Google Scholar]
- 37.Kanel KT, Wolmark N, Thompson PD. Delayed severe hypertriglyceridemia from tamoxifen. N Engl J Med. 1997;337:281. doi: 10.1056/NEJM199707243370417. [DOI] [PubMed] [Google Scholar]
- 38.Bruno S, Maisonneuve P, Castellana P, et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330:932. doi: 10.1136/bmj.38391.663287.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guvakova MA, Surmacz E. Tamoxifen interferes with the insulin-like growth factor I receptor (IGF-IR) signaling pathway in breast cancer cells. Cancer Res. 1997;57:2606–10. [PubMed] [Google Scholar]
- 40.Kleinman D, Karas M, Danilenko M, et al. Stimulation of endometrial cancer cell growth by tamoxifen is associated with increased insulin-like growth factor (IGF)-I induced tyrosine phosphorylation and reduction in IGF binding proteins. Endocrinology. 1996;137:1089–95. doi: 10.1210/endo.137.3.8603578. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal N, Sharma BC. Insulin resistance and clinical aspects of non-alcoholic steatohepatitis (NASH) Hepatol Res. 2005;33:92–6. doi: 10.1016/j.hepres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Best L. Inhibition of glucose-induced electrical activity by 4-hydroxytamoxifen in rat pancreatic beta-cells. Cell Signal. 2002;14:69–73. doi: 10.1016/s0898-6568(01)00223-6. [DOI] [PubMed] [Google Scholar]
- 43.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. Increased prevalence of prior breast cancer in women with newly diagnosed diabetes. Breast Cancer Res Treat. 2006;98:303–9. doi: 10.1007/s10549-006-9166-3. [DOI] [PubMed] [Google Scholar]
- 44.Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–8. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 45.Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous Steroid Hormone Concentrations and Risk of Breast Cancer Among Premenopausal Women. J Natl Cancer Inst. 2006;98:1406–15. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 46.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–65. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 47.Micheli A, Muti P, Secreto G, et al. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer. 2004;112:312–8. doi: 10.1002/ijc.20403. [DOI] [PubMed] [Google Scholar]
- 48.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein L, Deapen D, Cerhan JR, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–62. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 50.Decensi A, Maisonneuve P, Rotmensz N, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111:650–6. doi: 10.1161/01.CIR.0000154545.84124.AC. [DOI] [PubMed] [Google Scholar]