Abstract
Background
Regional differences in meal fat storage may explain preservation of fat accumulation in obese individuals.
Objectives
To determine whether meal fatty acid (FA) metabolism differs by sex and obesity phenotypes before and after weight loss.
Design
A [3H]triolein containing meal was given to trace meal FA oxidation (3H2O generation) and adipose tissue uptake (abdominal subcutaneous and gluteal biopsies) in 13 upper body obese (UOb) men, 9 UbO and 8 lower body obese (LOb) women (study 1). Fat distribution was measured using DXA and an abdominal CT. The volunteers participated in a diet/exercise weight loss program, after which 23 returned for an identical study 2.
Results
Study 1 - Storage of meal FA (mg meal fat/g adipose lipid) was greater in gluteal than abdominal fat (p=0.022) in LOb women, but not in UOb women and men. UOb men stored a lesser % of meal FA in both upper and lower body subcutaneous fat than LOb and UOb women (p=0.001 and p=0.044, respectively). Study 2 - participants returning for study 2 had lost 14.1 ± 1.1 kg. Changes in uptake of meal FA followed a pattern indicative of obesity phenotype maintenance by group as uptake of meal FA in upper body subcutaneous fat increased (p=0.028) in weight reduced UOb women and men (p=0.046) and decreased in lower body fat (p=0.025) in UOb men.
Conclusion
The differences in meal FA trafficking by obesity phenotype suggests that meal FA storage may play a role in regulating body fat distribution of obese individuals.
Keywords: Body fat distribution, indirect calorimetry, 3H triolein, adipose biopsy
Introduction
An android or upper body fat distribution is an important factor in predicting the increased health risks of obesity (1-3). A wide range of fat distribution can be seen in both adult men and women, although men typically store fat in the upper body and women in the lower body. Why individuals vary so much in accumulating fat in different regions is largely unknown. In order for some depots to expand to a greater extent than others there must be regional differences in adipocyte metabolism, either in lipolysis or fatty acid uptake. The available evidence suggests that regional variations in lipolysis (e.g. defective lipolysis in the larger depots) do not explain fat distribution (4-7), implying that differences in fatty acid uptake could be important in determining body fat patterning. Initial studies in normal weight men and women have not shown that storage of meal derived fatty acids explain differences in body fat accumulation and maintenance of distribution in men and women (8-11). These studies however, do not rule out the possibility that in obese individuals with certain obesity phenotypes, meal fat storage may play a role in preserving fat distribution. If meal fat metabolism helps to maintain regional body fat distribution, this role may differ among men and women. Whether regional differences in meal fat storage may explain preservation of preferential fat accumulation in obese individuals has yet to be investigated.
The single greatest source of fat for adipose storage is dietary triglyceride. We (8, 10, 12-14) and others (9, 15, 16) have examined whether regional differences in dietary fat storage relate to body fat distribution in a manner that suggests a cause and effect relationship. This is done by measuring meal fatty acid uptake using the meal fatty acid tracer/adipose biopsy technique pioneered by Mårin et al (9, 17). Herein we present the results of studies that use this approach to examine how meal triglyceride fatty acid storage into adipose tissue lipid varies by sex and obesity phenotype.
Weight loss improves the co-morbidities of obesity such as hypertension, dyslipidemia, insulin resistance and T2DM (18-20). However, weight loss has also been reported to alter substrate oxidation in a manner that favors lipid storage (21, 22), perhaps via changes in lipoprotein lipase (LPL) activity (23). Whether these weight loss effects are related to how dietary fat is trafficked into storage or oxidative pathways, and whether the effects vary by obesity phenotype is unknown. To the extent that altered fat oxidation is related to the tendency to regain weight, an understanding of how dietary fatty acid metabolism changes with weight loss might help us develop more effective ways of treating obesity.
The primary objective of this study was to determine whether meal fatty acid oxidation and uptake into subcutaneous adipose tissue varies by sex and body fat distribution. We also examined how weight loss through diet and exercise affect meal fatty acid trafficking in these same groups.
Methods
Subjects
Thirty volunteers provided written, informed consent to participate in the study. All participants were obese (BMI > 30 kg/m2) and weight stable, defined as weight ± 1.0 kg, for at least 2 months before the trial. All volunteers were sedentary or lightly active previous to participating in the study as defined by the American College of Sports Medicine (24). Women with a waist-to-hip ratio ≥ 0.85 or ≤ 0.79 were recruited to create two groups identified as upper body obese (UOb) and lower body obese (LOb), respectively. All men recruited were upper body obese (WHR ≥ 0.95). A normal complete blood count, serum creatinine, liver function tests, and electrolytes were required. All female subjects had to be non-pregnant before and during the study periods. No medications that affect fatty acid metabolism were permitted during the studies and none of the women were taking oral contraceptives.
Materials and Assays
Fat free mass (FFM), total body and leg fat mass (FM) were measured using dual-energy x-ray absorptiometry (DXA) (DPX-IQ, Lunar Radiation; Madison, WI) (25). Abdominal subcutaneous and visceral adipose tissue areas were determined by a single-slice abdominal computed tomography (CT) scan at the level of L2-3. The CT data, combined with DXA determined total abdominal fat content, was used to calculate visceral fat mass (26).
Resting metabolic rate (RMR), and substrate oxidation was assessed via indirect calorimetry using a DeltaTrac Metabolic Cart (Yorba Linda, CA) after a 12 h overnight fast while volunteers were in a steady state (27). After the experimental meal (see below) measurements were taken every 30 minutes over the following 5 hours. Steady state was achieved with the initial indirect calorimetry measurement that was 30 minutes in duration with a 5 minute acclimatization period. Each indirect calorimetry measurement thereafter was 10 minutes in duration which included 5 minutes to establish steady state. Total body water was measured using H2 18O (28).
Protocol
The meal fatty acid metabolism studies were conducted in the Mayo Clinical Studies Unit (CSU) before (study 1) and after (study 2) the weight loss intervention. Body composition and total body water was measured before the inpatient studies. To isolate the effects of weight loss on fat oxidation and meal fat deposition, the participants were required to remain weight stable for at least 1 month and consume meals provided by the CSU for 7 days before the meal tracer studies. The provision of meals ensured that the participants in each group were in an isocaloric state before being studied. The diet provided energy in the form of 35% fat, 50% carbohydrate, and 15% protein. Amount of energy intake required for weight maintenance was estimated on the basis of measured resting energy expenditure by indirect calorimetry multiplied by an activity factor of 1.5 with subsequent adjustments to maintain weight stability. Volunteers were required to refrain from vigorous exercise for 2 days prior to admission, which was the evening before the meal tracer study.
Weight loss in between the study phases of the trial was achieved through a diet and exercise regimen where subjects were instructed to decrease caloric intake and increase energy expenditure in an amount that would create a 500 kcal/d deficit. The weight loss diet consisted of 20-25% fat, 20% protein, and 55-60% carbohydrate. All food was provided by the CSU for the first 4 weeks. During the initial 4 week period, participants were taught dietary principles, and weight management and behavioral training techniques. After this 4 week period, subjects selected, prepared, and consumed food at home. The exercise program started with 15 minutes of aerobic exercise three times per week at 50% of heart rate reserve (HRR), defined as the difference between resting and maximal heart rate. After the initial 4 week period, participants were asked to gradually increase their activity to 45 min aerobic exercise four times a week at 60-70% of HRR. The exercise program was not supervised and adherence was self reported. Volunteers were weighed weekly at the CSU.
Plasma FFA and insulin
Baseline FFA and postabsorptive insulin was measured using four samples collected before the test meal. Postprandial insulin was calculated from the average of four and five blood samples, respectively collected after the test meal. Plasma free fatty acids were measured using high-performance liquid chromatography. Insulin was measured using a radioimmunoassay.
Meal uptake and oxidation
At 0800 the morning of the study the volunteer consumed a liquid meal (Ensure Plus, Ross Laboratories). The test meal provided 1/3 of the resting energy expenditure in UOb men and 1/3 of the total energy expenditure in LOb, and UOb women. The test meals were selected in this manner so as to avoid huge differences in the energy and fat content of the meals between men and women. The meal provided 835±15 kcal, 856±24 kcal, and 750±29 kcal for LOb women, and UOb women and men, respectively. The macronutrient distribution and energy content of the experimental meal was assessed according to the volume consumed (to the nearest 5 ml) and the manufacturers listed contents. The energy content of the meals for participants who completed both studies was 793±21 kcal before weight loss and 688±21 kcal after weight loss. The energy content of the post-weight loss test meal was modified from the initial meal for each volunteer on an individual basis based upon any changes in resting metabolic rate between the first and second study. The experimental meal contained 57% carbohydrate, 27% fat, and 15% protein. 40 μCi of [3H]triolein was sonicated into the meal just prior to consumption. Quadruplicate 50 μl samples of the test meal were counted on a liquid scintillation counter to determine the exact amount of [3H]triolein consumed. Lunch and supper were given at 1300 and 1800 h and consisted of solid foods with the same macronutrient composition as those provided during the week prior to admission. During the first 8 h after the test meal, volunteers were required to remain in bed except as needed to void. Urine was collected for 24 h after the test meal to assess 3H2O losses and after completely voiding the morning after the test meal a second sample of fresh urine was collected and analyzed for 3H2O concentration.
Biopsies of subcutaneous adipose tissue (SAT) from the abdominal and gluteal regions were obtained at 0800 hr the next day using sterile technique under local anesthesia. Lipid was extracted from the tissues, weighed and counted on the scintillation counter to <2% error. Specific activity (SA) was then calculated for each site. The 40 μCi dose of [3H]triolein resulted in a typical SA of 500-750 dpm/g lipid. For study 2, abdominal and gluteal SAT biopsies were performed the day before the test meal to determine baseline adipose lipid SA, which was subtracted from the adipose lipid SA in tissue obtained following the study 2 test meal. The percent of meal FA stored in lower and upper body SAT were then calculated by the following equation:
The following equation was used to calculate the percentage of meal fat oxidized:
#Activity of total body water was determined by plasma SA (dpm/mL) and total body water (mL).
Typically, the sum of the meal FA oxidized and stored does not account for all meal FA consumed. Calculation of percent unaccounted for meal fat is as follows:
Substrate Oxidation
The amount of energy expended the morning of the study was determined by indirect calorimetry (27). Before and after the test meal was consumed, v̇O2 and V̇CO2 were measured for 15 minutes at 0, 30, 60, 90, 120, 180, 240, 300 minutes. Because participants were weight stable prior to and during the study, all subjects were assumed to be in nitrogen balance (nitrogen intake = nitrogen losses) and therefore urine nitrogen losses for the purposes of calculating substrate oxidation were considered to be equal to nitrogen intake. Carbohydrate and fat oxidation values at each time point were calculated and total oxidation of each substrate was determined by area under the curve (g) from 0 to 300 minutes.
Statistics
All data were tested for normal distribution with Shapiro-Wilk test for normality. One-way analysis of variance (ANOVA) with a posthoc Tukey test was used to evaluate statistically significant differences in study 1 baseline characteristics, body composition, meal fatty acid uptake, and oxidation between groups. Analysis of covariance (ANCOVA) was use to examine whether obesity phenotypes or sex differences predicted meal fatty acid oxidation using FFM as a covariate. ANCOVA with posthoc Tukey HSD was used to compare whether changes in FFA, insulin, meal fatty acid uptake and oxidation were different between groups after weight loss using baseline values as a covariate. Paired t-tests were used to determine whether statistically significant within group differences exist in meal uptake and oxidation between depots, and in body composition after weight loss. Pearson’s correlations were used to evaluate how body composition relates to measured meal FA parameters before and after weight loss. As the number of hypotheses that were not a priori was limited, no statistical adjustments were made for multiple comparisons. Data were analysed using SPSS for Windows (version 12.0.0; SPSS Inc., Chicago, IL) and JMP (version 7.0.1; SAS Institute, Cary, NC). Data are presented as mean ± SEM. Differences were defined as statistically significant at p<0.05.
Results
Study 1
Subject characteristics
Characteristics of the volunteers participating in study 1 are provided in Table 1. Of the 30 participants, 9 were UOb women, 13 were UOb men, and 8 were LOb women. The groups were well matched for age and body mass index (BMI). Resting energy expenditure was greater (p<0.001) in men than women. The expected patterns of differences in total and regional fat mass (visceral, leg and upper body SAT) were present.
Table 1.
Subject characteristics and meal study data for participants completing study 1
| Lower body obese women (n=8) | Upper body obese women (n=9) | Upper body obese men (n=13) | |
|---|---|---|---|
| Age (y) | 39±2 | 40±2 | 42±1 |
| Basal Metabolic Rate (kcal/day) | 1628±68a | 1781±65a | 2057±51b |
| BMI (kg/m2) | 33.6±0.6 | 33.8±0.9 | 33.0±0.8 |
| Weight (kg) | 91.5±3.0a | 93.8±3.0a | 104.1±2.8 b |
| % Fat | 52.1±0.9a | 46.1±1.4a | 32.1±1.6 b |
| VAT (kg) | 5.3±0.6a | 6.6±0.5a,b | 8.1±0.6b |
| Lower body SAT(kg) | 17.4±1.6a | 14.3±1.2a,b | 10.6±0.8b |
| Upper body SAT (kg) | 25.1±1.1a | 22.5±1.8a | 14.7±1.1 b |
| Plasma FFA & Insulin: | |||
| Postabsorptive FFA (μmol/L) | 617±33 | 730±40 | 724±47 |
| Postabsorptive insulin (pmol/L) | 44±5 | 91±9 | 67±18 |
| Postprandial insulin (pmol/L) | 264±42 | 245±70 | 411±63 |
| Whole body 5 h substrate utilization (g): | |||
| Carbohydrate | 29.3±5.4 | 39.8±5.4 | 40.8±5.2 |
| Fat | 15.5±0.9 | 15.3±1.8 | 24.8±2.5 |
| Meal FA storage (mg meal fat/g lipid): | |||
| Abdominal SAT | 0.38±0.041 | 0.24±0.04 | 0.17±0.02 |
| Gluteal SAT | 0.62±0.092 | 0.41±0.10 | 0.20±0.03 |
| Meal FA storage/utilization (%): | |||
| Upper body SAT | 20.3±1.0a | 18.1±3.3a | 7.1±1.2 b |
| Lower body SAT | 22.8±7.8a | 15.2±4.3a | 6.0±3.2 b |
| Oxidation | 18.5±4.3 | 34.3±8.8 | 36.5±3.2 |
| Unaccounted for | 38.3±4.0 | 34.4±11.4 | 50.5±4.0 |
Data are shown as mean ± SEM. Means with different superscript letters (e.g. a,b) indicate significant differences within the same row at p<0.05 as per one-way ANOVA with posthoc Tukey tests. Means with different superscript numbers (e.g. 1,2) indicate significant differences between depots at p<0.05 as per paired t-test.
Substrate and meal fatty acid oxidation
For the 5 h after the experimental meal, there were no differences in carbohydrate (p = 0.47) or fat oxidation (p = 0.73) between groups (Table 1). There were no significant between group differences in 24 h meal FA oxidation (p = 0.32). No variations were found in postabsorptive insulin levels (p = 0.14), postprandial insulin levels (p = 0.15) or postabsorptive FFA concentrations (p = 0.18).
Meal fatty acid storage in adipose tissue
In LOb women gluteal adipose stored more (p=0.022) meal FA than abdominal adipose tissue (on a mg meal FA/g adipose lipid basis). No such site differences were detected in UOb women or men (Table 1).
UOb men stored a lesser % of meal FA in both upper and lower body subcutaneous fat than LOb and UOb women (p=0.001 and p=0.044, respectively). The percent of meal fat stored in upper body vs. lower body subcutaneous depots within the same group was not significantly different (p = 0.23 UOb men, p = 0.45 LOb women, and p = 0.55 UOb women). There were no differences (p = 0.44 UOb men vs. LOb women, p = 0.93 LOb vs. UOb women, and p = 0.22 UOb men vs. UOb women) in unaccounted for meal fat between groups.
Relationships between regional fat distribution and meal FA metabolism
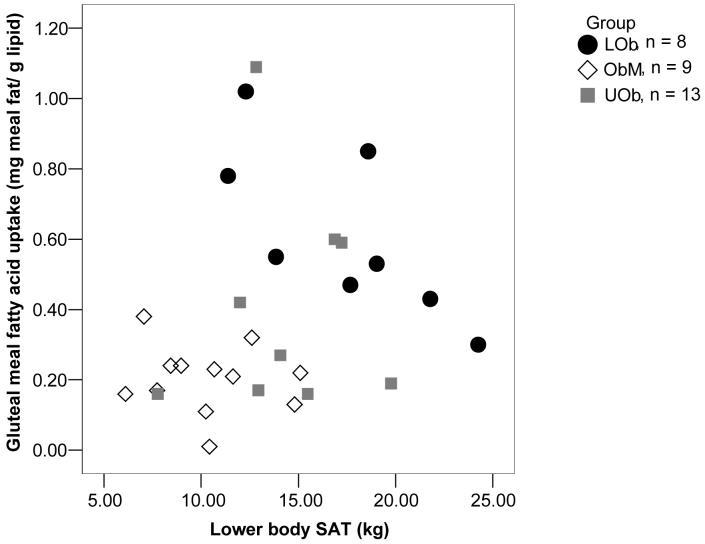
We observed a trend (p = 0.051) for a positive association (r = 0.37) between abdominal meal FA storage and upper body subcutaneous fat mass. For the combined group, meal FA storage in gluteal fat (mg meal fat/g adipose lipid) increased in relation to lower body fat mass (Figure 1). In contrast, the amount of lower body fat was inversely associated with the storage of meal fat (mg meal fat/g of gluteal adipose lipid, r = -0.74; p=0.037) in LOb women, however, this was not a pre-specified hypothesis to be tested in the LOb women alone.
Figure 1.
Study 1-Lower body SAT vs. gluteal meal fatty acid uptake 24 h after ingestion of a test meal (n=30) plotted for each group. The positive relationship (r=0.25) in the group as a whole was not significant (p=0.20). No relationship was seen between lower body SAT and gluteal meal fatty acid uptake in UOb women and obese men. In LOb women, a negative correlation was observed between lower body SAT and gluteal meal FA uptake (r=-0.74; p=0.037) as per Pearson correlation.
Study 2
Subject characteristics
Twenty-three volunteers returned for the post-weight loss study (study 2). The characteristics of the participants in study 2 are provided in Table 2, sorted by obesity sub-group. The average age of those returning for study 2 was 42 ± 1 y and there were no significant differences between ages (p=0.20) or initial BMI (p=0.77) of UOb men, LOb or UOb women in study 2. Seven participants did not return to participate in study 2 because they were unable to comply with the study protocol and had difficulty attending scheduled study visits. There were no differences in baseline characteristics between participants who completed study 2 and those who did not.
Table 2.
Characteristics of subjects who completed Study 2 before and after weight loss by group
| Lower body obese women (38±6 y; n=5) | Upper body obese women(42±5 y; n=6) | Upper body obese men (43±3 y; n=12) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | Before | After | Change | Before | After | Change | |
| BMR (kcal) | 1589±74 | 1470±68 | 118±112 | 1756±84 | 1677±119 | 79±73 | 2054±51 | 1760±84 | 295±63 |
| BMI (kg/m2) | 33.4±0.9 | 28.7±1.4 | -4.7±1.2* | 33.2±1.0 | 29.3±0.9 | -3.9±0.5* | 32.6±0.7 | 27.3±0.6 | -5.3±0.6* |
| Weight (kg) | 88.0±3.8a | 75.6±4.4 | -12.4±3.4* | 95.7±4.0a,b | 83.8±3.6 | -11.9±1.4* | 103.1±2.9b | 87.2±2.7 | -16.0±1.5* |
| %Fat | 50.9±0.5a | 45.6±1.61 | -5.3±1.3* | 47.1±1.8a | 43.3±2.01 | -3.7±0.6* | 31.4±1.6b | 25.8±1.72 | -5.6±2.3* |
| VAT (kg) | 5.1±0.7a | 3.3±0.31 | -1.8±0.5* | 6.9±0.7a,b | 5.8±0.22 | -1.1±0.6 | 8.3±0.6b | 5.2±0.61 | -3.0±0.7* |
| Lower body SAT(kg) | 14.8±1.4a | 13.3±1.11 | -1.4±1.1 | 14.5±1.7a | 12.3±1.21 | -2.3±1.0 | 10.2±0.8b | 6.8±0.62 | -3.4±0.8* |
| Upper body SAT (kg) | 25.0±1.6a | 18.1±2.11 | -6.9±2.5 | 23.8±2.4a | 18.5±1.61 | -5.4±1.6* | 13.9±0.8b | 10.6±0.82 | -3.3±1.0* |
Data are shown as mean ± SEM. Means with different superscript letters (e.g. a,b) indicate significant differences in baseline characteristics at p<0.05 as per one-way ANOVA with posthoc Tukey tests. Means with different superscript numbers (e.g. 1,2) indicate significant differences in after characteristics at p<0.05 as per one-way ANOVA with posthoc Tukey tests. Means with an asterisk (*) denote a significant change after weight loss at p<0.05 as per paired t-tests.
Meal fatty acid oxidation after weight loss
Overall, the amount of carbohydrate oxidized during the 5 h post-meal interval did not change in participants following weight loss (34.2 ± 3.3 g vs. 40.6 ± 3.3 g; p = 0.15), however, postprandial fat oxidation declined (20.0 ± 1.8 g vs. 15.2 ± 1.6 g; p = 0.016) after weight loss. There was no difference (p = 0.15) in change in the amount of meal fat oxidized after 24 h following weight loss (7.69 ± 0.79 g vs. 9.33 ± 0.65 g). Percent of meal FA oxidized in the 24 h after the experimental meal increased (p = 0.014) after the diet and exercise program resulting in weight loss (29.2 ± 3.0 % vs. 40.7 ± 2.8 %).
Plasma FFA and insulin concentrations (Table 3)
Table 3.
Study 2 - Meal fat storage and oxidation, plasma FFA and insulin before and after weight loss by group
| Lower body obese women (n=5) | Upper body obese women (n=6) | Upper body obese men (n=12) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | Before | After | Change | Before | After | Change | |
| Test meal: | |||||||||
| Energy (kcal) | 843±23 | 755±35 | -88±26* | 840±30 | 771±32 | -70±14* | 748±31 | 618±18 | -130±33* |
| Fat (g) | 28.4±0.8 | 25.4±1.2 | -3.0±0.9* | 28.3±1.0 | 25.9±1.1 | -2.4±0.5* | 25.2±1.1 | 20.8±0.6 | -4.4±1.1* |
| Plasma FFA & Insulin: | |||||||||
| Postabsorptive FFA (μmol/L) | 631±45 | 447±52a,b | -184±37* | 726±38 | 580±52a | -146±73 | 718±50 | 440±27b | -278±48* |
| Postabsorptive insulin (pmol/L) † | 42±7 | 28±5 | -14±5 | 84±14 | 81±31 | -2±27 | 74±23 | 57±14 | -17±10 |
| Postprandial insulin (pmol/L) † | 269±37 | 136±11 | -133±60* | 403±96 | 389±131 | -14±191 | 256±77 | 462±129 | 206±109 |
| Whole body 5 h substrate utilization (g): ‡ | |||||||||
| Carbohydrate | 22.4±1.6 | 32.3±6.2 | 9.9±6.7 | 38.0±6.0 | 38.6±3.6 | 0.6±4.0 | 37.6±5.1 | 45.5±5.3 | 7.8±7.8 |
| Fat | 14.8±1.2 | 10.4±2.9 | -4.4±2.2 | 14.6±2.3 | 13.5±1.1 | -1.1±19 | 25.3±2.5 | 18.4±2.7 | -6.9±3.3 |
| Meal 24 h FA oxidation (g): | |||||||||
| 5.14±1.62 | 8.94±2.45 | 3.80±3.29 | 7.30±2.01 | 10.79±0.84 | 3.49±2.34 | 8.94±0.81 | 8.76±0.65 | -0.18±1.03 | |
| Meal FA storage (mg meal fat/g lipid): | |||||||||
| Abdominal SAT | 0.42±0.06 | 0.53±0.19 | 0.11±0.17 | 0.19±0.03 | 0.31±0.05 | 0.12±0.04* | 0.17±0.02 | 0.20±0.051 | 0.04±0.06 |
| Gluteal SAT | 0.73±0.10 | 0.84±0.18 | 0.10±0.17 | 0.26±0.07 | 0.45±0.14 | 0.20±0.12 | 0.20±0.03 | 0.14±0.042 | -0.05±0.05 |
| Meal FA storage/utilization (%): | |||||||||
| Upper body SAT | 19.6±0.5 | 21.6±4.1 | 2.0±3.5 | 19.0±4.9 | 18.5±4.9 | -0.5±8.0 | 6.2±0.8 | 10.0±1.91 | 3.8±5.8* |
| Lower body SAT | 21.4±4.3 | 28.4±4.3a | 7.0±6.2 | 12.0±5.8 | 16.5±4.9a,b | 4.5±6.9 | 5.5±0.8 | 5.2±1.3b, 2 | -0.3±1.2 |
| Oxidation | 18.4±5.9 | 35.6±10.0 | 17.2±13.2 | 25.2±6.3 | 42.2±4.7 | 17.0±8.8 | 35.8±3.4 | 42.1±3.0 | 6.3±4.7 |
| Unaccounted for | 40.4±5.3 | 14.4±9.2a | -26.0±30.7 | 47.0±10.5 | 22.8±7.9a,b | -24.2±14.5 | 52.7±3.6 | 42.8±4.7b | -9.9±6.1 |
Data are shown as mean ± SEM. Means with an asterisk (*) denote a significant change after weight loss at p<0.05 as per paired t-tests. Means with different superscript letters (e.g. a,b) indicate significant differences in change after weight loss between groups at p<0.05 as per ANCOVA with baseline values used as a covariate and posthoc Tukey tests. Means with different superscript numbers (e.g. 1,2) indicate significant differences in change between depots at p<0.05 as per paired t-tests. N.B.
In LOb women n = 4; in UOb women n = 5; in obese men n = 10.
In obese men n = 11: Test meal composition for energy and fat respectively is before: 758±33 kcal & 25.5±1.1 g and after: 616±19 kcal & 20.7±0.6 g.
For the group as a whole, postabsorptive plasma FFA concentrations decreased (p < 0.001) after weight loss (701 ± 30 μmol/L vs. 478 ± 25 μmol/L). There were no significant changes (p = 0.15 and p = 0.34, respectively) in postabsorptive or postprandial plasma insulin concentrations following weight loss (70 ± 13 pmol/L vs. 57 ± 11 pmol/L and 302 ± 49 pmol/L vs. 375 ± 78 pmol/L, respectively).
When examined as sub-groups, decreases in postabsorptive FFA concentrations were found in LOb women (p = 0.007) and UOb men (p < 0.001) (Table 3). Postprandial insulin concentrations decreased (p = 0.02) following weight loss in LOb women.
No differences were found in the changes of substrate and meal fatty acid oxidation or postabsorptive plasma insulin concentrations from study 1 to study 2 in LOb women, UOb women or UOb men. In comparing the changes between groups, there was a difference in changes in postabsorptive FFA (p = 0.049), with a greater decrease in UOb men (-278 ± 31 μmol/L) than UOb women (-145 ± 44 μmol/L). No between group differences were found in any of the other above mentioned parameters (Table 3).
Meal fatty acid storage after weight loss
Neither upper body nor lower body subcutaneous meal FA storage changed after the diet and exercise program resulting in weight loss (0.23 ± 0.14 vs. 0.31 ± 0.27 mg meal fat/g lipid; p = 0.32, and 0.34 ± 0.27 vs. 0.40 ± 0.39 mg meal fat/g lipid; p = 0.28, respectively) for the combined group of men and women. Within the obesity subgroups, however, meal FA storage increased in UOb women after weight loss, significantly (p = 0.028) so in abdominal fat (see Table 3). Because both groups of obese women also lost substantial amounts of subcutaneous fat, the percent of meal FA storage in upper and lower body subcutaneous fat did not change significantly from study 1 to study 2 (Table 3). In UOb men, we observed a significant increase (p<0.05) in the percent of meal FA storage in abdominal subcutaneous adipose tissue from study 1 to study 2 and a small, but significant (p<0.05) decrease in meal FA storage in lower body fat (Table 3). A difference (p = 0.004) was found between changes in the percentage of meal fat stored in lower body SAT among groups after weight loss where change in percent meal fat stored in lower body SAT in LOb women (7.2 ± 4.3 %) was different than the change in UOb men (-0.2 ± 2.6 %).
After weight loss the proportion of meal FA that was unaccounted for decreased for the group as a whole (48.5 ± 3.5 % vs. 31.4 ± 4.4 %; p=0.006), but there were no significant changes within the sub-groups. Between groups, a difference (p = 0.01) in change of unaccounted for meal fat was seen after weight loss where the change in LOb women (-25.8 ± 8.2 %) was larger than in UOB men (-9.9 ± 5.2 %).
Relationships between changes in regional fat distribution and meal FA metabolism after weight loss
No statistically significant relationships were detected between changes in regional fat distribution and meal FA metabolism after weight loss in the group as a whole. The change in the storage of meal FA (mg/g adipose lipid) tended (r = -0.40, p=0.059) to increase as a function of the loss of body fat (change in percent body fat), which would not be surprising in that the same relative amount of dietary fat might be stored in less body fat.
In UOb men, decreases in fat mass were associated (p=0.008 for both lower and upper body fat) with more meal FA storage in abdominal and gluteal SAT (r=-0.72 for both). In addition, meal FA storage per g abdominal adipose lipid after the diet and exercise program resulting in weight loss was negatively correlated (r=-0.65; p=0.022) with changes in upper body subcutaneous fat mass in UOb men. In UOb men the changes in lower body fat after weight loss were also negatively associated (r=-0.77; p=0.003) with meal storage per g gluteal adipose lipid.
Discussion
This study was designed to test whether obese men and women with different body fat distributions would have patterns of meal FA trafficking consistent with their fat patterning. If so, this might suggest that meal fat storage contributes to differences in body fat distribution in obesity. We also sought to understand how meal FA metabolism is affected by a diet and exercise program resulting in weight loss. We found that gluteal fat concentrated meal FA to a greater extent than abdominal subcutaneous fat in LOb women, but this was not the case in UOb women or men. We also observed that, although UOb men stored the same portion of dietary FA in upper and lower body subcutaneous depots under weight stable conditions, they stored a significantly greater portion of dietary fat in upper body SAT than in lower body SAT following weight loss. Thus, in obese adults, storage of dietary fat in different adipose depots may contribute to variations in body fat distribution.
We found it interesting that LOb women stored more meal FA in lower body fat than upper body fat. The finding of preferential storage of meal FA in lower body fat contrasts with previous reports that meal FA storage (mg meal fat/g adipose lipid) is greater in upper body than lower body subcutaneous fat in normal weight men and women (9-11, 17). A recent study of meal FA uptake in obese women did not detect any differences in lower and upper body meal FA storage (13), although there was a significant trend for meal FA storage in leg fat to increase in relation to leg fat mass in women receiving a normal fat meal. Our finding that LOb women stored significantly more meal fat in lower body than upper body adipose tissue is consistent with that trend. This implies that in some individuals lower body adipose tissue has a competitive advantage relative to upper body adipose tissue, and that this advantage works to create and/or maintain a lower body obesity phenotype. Although another possibility is that accelerated lipolysis in lower body fat cells in LOb women is offset by preferential uptake of meal FA, in vivo measures of leg FFA release have consistently demonstrated that lower body fat is “hypo-lipolytic” relative to upper body fat (4, 6, 29). The corollary to this meal fat storage hypothesis is that individuals in whom upper body fat is more efficient in storing dietary fat will develop an upper body fat distribution along with the associated health risks.
We also found evidence in favor of the role of meal FA storage may have in maintaining sexual dimorphism of fat distribution. Much less meal FA storage was found in subcutaneous fat in UOb men, both lower and upper body, than in obese women. This pattern in obese individuals was more pronounced than that observed in normal weight men and women (10, 11).
For the groups combined, we observed an increase in the 24 h dietary fat oxidation following weight loss despite a slight reduction in total fat oxidation for the 5 h after the experimental meal. This suggests that after a diet and exercise program resulting in weight loss more dietary fat is used for immediate energy needs, which seems to be more than offset by a reduction in the oxidation of endogenous fatty acids. The difference in 5 h and 24 h fat oxidation could be due to enhanced insulin-mediated suppression of FFA availability (30), which in turn may contribute to the improved tissue sensitivity to insulin following weight loss (31-33). Though changes (or lack thereof) in postabsorptive and postprandial insulin levels may not be accurate indicators of insulin sensitivity, there was a significant overall decrease in postabsorptive plasma FFA concentrations after weight loss. The changes in FFA concentrations with weight loss may indicate that the reduced total fatty acid oxidation despite overall increases in 24 h meal fat oxidation are a reflection of better regulation of adipose tissue lipolysis.
After losing significant amounts of body fat, there was an expected pattern of increasing meal fat uptake (mg meal FA/g adipose lipid), especially in upper body fat in UOb women and men. This suggests that abdominal fat cells in these individuals may respond to losses in intracellular TG by enhancing the storage of dietary fat in this region, and would likely contribute to preferential re-gain of upper body fat after dieting. Similarly, after weight loss there was a significant difference in the changes of percent lower body meal fat uptake between LOb women and men where lower body percent meal fat storage increased in women and slightly decreased in UOb men. The preferential sequestering of fat into lower body depots in lower body obese women further supports a role of dietary fat in maintaining regional body fat distribution.
There are a number of potential explanations for the regional differences in meal FA storage in LOb women and for the sex differences in meal FA storage. These range from differences in regional, postprandial adipose tissue blood flow (10, 34, 35), LPL activity (15, 36, 37), cellular fatty acid transport (38) or efficiency of adipocyte TG synthesis (39). Previous studies that compare regional LPL levels in men and women have generally found higher levels of LPL in women than men (15, 36, 37). Furthermore, lower body obese women have been reported to have higher LPL activity in gluteal and femoral fat implying greater potential for FA uptake in these regions (40). However, LPL activity cannot completely account for sex differences in regional fat uptake (41, 42). Contrary to what might be expected, a 10 kg of weight loss was shown to reduce LPL activity in upper and lower body fat in women, but not in men (15). Little is known about the relative contributions of blood flow, fatty acid transport or TG synthesis steps as regulators of regional meal fatty acid storage. Unfortunately, beyond describing the relationship between meal FA storage and body fat distribution in this study, we were not able to measure processes related to these steps.
The present study sampled gluteal subcutaneous fat to examine lower body meal fat uptake. Fat metabolism in gluteal subcutaneous fat has previously been observed to be intermediate between abdominal and thigh subcutaneous fat (10). In contrast, Uranga et al. (11) found no differences between meal FA uptake in gluteal and femoral subcutaneous fat regions. Despite the fact that thigh subcutaneous fat metabolism was not measured in the present study, abdominal and gluteal subcutaneous fat metabolism differed in a manner consistent to variations in upper and lower body obesity seen in previous literature. Thus, although we generally prefer thigh subcutaneous fat when examining difference between lower and upper body fat (10), gluteal subcutaneous fat appears to be acceptable in representing lower body fat metabolism (11).
In summary, we have studied how dietary fatty acids are stored in different subcutaneous fat depots in obese men and women with different distributions of body fat. The observation that women with LOb are more efficient at storing meal FA in gluteal than abdominal fat suggests that the trafficking of dietary triglyceride may play a role in determining fat distribution. In addition, UOb men store a much lower proportion of dietary fat in subcutaneous regions, which also hints at a role for this process in the sex differences in fat distribution. Finally, fat loss via diet/exercise modified meal FA storage in such a way as to favor maintenance of regional body fat distribution. Understanding the potential role of dietary fat trafficking in determining fat distribution will help focus future studies on more mechanistic processes.
Acknowledgments
Sylvia Santosa - analysis of data, writing of the manuscript, provision of significant advice; Susanne Votruba - analysis of data, writing of the manuscript, provision of significant advice; Donald Hensrud - collection of data, analysis of data, writing of the manuscript, Michael Jensen - design of the experiment, collection of data, analysis of data, writing of the manuscript. No conflict of interest is declared for any of the authors.
Supported by grants DK45343, DK40484, DK50456 and R00585 from the U.S. Public Health Service and by the Mayo Foundation.
References
- 1.Björntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 2.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF. The association of body fat distribution with hypertension, hypertensive heart disease, coronary heart disease, diabetes and cardiovascular risk factors in men and females aged 18-79 years. J Chron Dis. 1987;40:421–428. doi: 10.1016/0021-9681(87)90175-5. [DOI] [PubMed] [Google Scholar]
- 4.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88:609–613. doi: 10.1172/JCI115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest. 1995;96:2297–2303. doi: 10.1172/JCI118285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab. 2001;280:E1000–E1006. doi: 10.1152/ajpendo.2001.280.6.E1000. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab. 2003;285:E1282–E1288. doi: 10.1152/ajpendo.00220.2003. [DOI] [PubMed] [Google Scholar]
- 9.Marin P, Rebuffe-Scrive M, Bjorntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest. 1990;20:158–165. doi: 10.1111/j.1365-2362.1990.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 10.Romanski SA, Nelson R, Jensen MD. Meal fatty acid uptake in adipose tissue: Gender effects in non-obese humans. Am J Physiol Endocrinol Metab. 2000;279:E455–E462. doi: 10.1152/ajpendo.2000.279.2.E455. [DOI] [PubMed] [Google Scholar]
- 11.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab. 2005;288:E547–E555. doi: 10.1152/ajpendo.00340.2004. [DOI] [PubMed] [Google Scholar]
- 12.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo--relation to body fat distribution. Diabetes. 2007;56:1369–1375. doi: 10.2337/db06-1680. [DOI] [PubMed] [Google Scholar]
- 13.Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in females. Diabetes. 2007;56:2589–2597. doi: 10.2337/db07-0439. [DOI] [PubMed] [Google Scholar]
- 14.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab. 2006;291:E1115–E1123. doi: 10.1152/ajpendo.00196.2006. [DOI] [PubMed] [Google Scholar]
- 15.Imbeault P, Almeras N, Richard D, Despres JP, Termblay A, Mauriege P. Effect of a moderate weight loss on adipose tissue lipoprotein lipase activity and expression: existence of sexual variation and regional differences. Int J Obes Relat Metab Disord. 1999;23:957–965. doi: 10.1038/sj.ijo.0801025. [DOI] [PubMed] [Google Scholar]
- 16.Bickerton AST, Roberts R, Fielding BA, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56:168–176. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- 17.Marin P, Oden B, Bjorntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80:239–243. doi: 10.1210/jcem.80.1.7829619. [DOI] [PubMed] [Google Scholar]
- 18.Oster G, Thompson D, Edelsberg J, Bird AP, Colditz GA. Lifetime health and economic benefits of weight loss among obese persons. Am J Public Health. 1999;89:1536–1542. doi: 10.2105/ajph.89.10.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santosa S, Demonty I, Lichtenstein AH, Cianflone K, Jones PJ. An investigation of hormone and lipid associations after weight loss in women. J Am Coll Nutr. 2007;26:250–258. doi: 10.1080/07315724.2007.10719608. [DOI] [PubMed] [Google Scholar]
- 20.Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–484. doi: 10.1002/j.1550-8528.1999.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 21.Eckel RH, Hernandez TL, Bell ML, et al. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr. 2006;83:803–808. doi: 10.1093/ajcn/83.4.803. [DOI] [PubMed] [Google Scholar]
- 22.Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57:35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Eckel RH, Yost TJ. Weight reduction increases adipose tissue lipoprotein lipase responsiveness in obese women. J Clin Invest. 1987;80:992–997. doi: 10.1172/JCI113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–873. doi: 10.1016/s0025-6196(12)60695-8. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–278. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 27.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 28.Schoeller DA, vanSanten E, Peterson DW, Dietz W, Jaspen J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr. 1980;33:2686–2693. doi: 10.1093/ajcn/33.12.2686. [DOI] [PubMed] [Google Scholar]
- 29.Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48:1586–1592. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 30.Shadid S, Jensen MD. Pioglitazone increases non-esterified fatty acid clearance in upper body obesity. Diabetologia. 2006;49:149–157. doi: 10.1007/s00125-005-0051-0. [DOI] [PubMed] [Google Scholar]
- 31.Rector RS, Warner SO, Lui Y, et al. Exercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;293:E500–E506. doi: 10.1152/ajpendo.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coughlin CC, Finck BN, Eagon JC, et al. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity. 2007;2007:3. doi: 10.1038/oby.2007.556. [DOI] [PubMed] [Google Scholar]
- 33.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasig energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes. 2002;51:2467–2473. doi: 10.2337/diabetes.51.8.2467. [DOI] [PubMed] [Google Scholar]
- 35.Summers LKM, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci. 1996;91:679–683. doi: 10.1042/cs0910679. [DOI] [PubMed] [Google Scholar]
- 36.Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes. 1987;11:129–140. [PubMed] [Google Scholar]
- 37.Arner P, Lithell H, Wahrenberg H, Bronnegard M. Expression of lipoprotein lipase in different human subcutaneous adipose tissue regions. J Lipid Res. 1991;32:423–429. [PubMed] [Google Scholar]
- 38.Bower JF, Davis JM, Hao E, Barakat HA. Differences in transport of fatty acids and expression of fatty acid transporting proteins in adipose tissue of obese black and white women. Am J Physiol Endocrinol Metab. 2006;290:E87–E91. doi: 10.1152/ajpendo.00194.2005. [DOI] [PubMed] [Google Scholar]
- 39.Ranganathan G, Unal R, Pokrovskaya I, et al. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res. 2006;47:2444–2450. doi: 10.1194/jlr.M600248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raison J, Basdevant A, Sitt Y, Guy-Grand B. Regional differences in adipose tissue lipoprotein lipase activity in relation to body fat distribution and menopausal status in obese women. Int J Obes. 1988;12:465–472. [PubMed] [Google Scholar]
- 41.Kolehmainen M, Vidal H, Ohisalo JJ, Pirinen E, Alhava E, Uusitupa MI. Hormone sensitive lipase expression and adipose tissue metabolism show gender difference in obese subjects after weight loss. Int J Obes Relat Metab Disord. 2002;26:6–16. doi: 10.1038/sj.ijo.0801858. [DOI] [PubMed] [Google Scholar]
- 42.Fielding BA, Frayn KN. Lipoprotein lipase and the disposition of dietary fatty acids. Br J Nutr. 1998;80:495–502. doi: 10.1017/s0007114598001585. [DOI] [PubMed] [Google Scholar]