Abstract
This study sought to increase current understanding of the neuro-psychological basis of poor reading ability by using fMRI to examine brain activation during a visual sentence comprehension task among good and poor readers in the third (n = 32) and fifth (n = 35) grades. Reading ability, age, and the combination of both factors made unique contributions to cortical activation. The main finding was of parietotemporal underactivation (less activation than controls) among poor readers at the 2 grade levels. A positive linear relationship (spanning both the poor and good readers) was found between reading ability and activation in the left posterior middle temporal and postcentral gyri and in the right inferior parietal lobule such that activation increased with reading ability. Different developmental trajectories characterized good and poor readers in the left angular gyrus: activation increased with age among good readers, a change that failed to occur among poor readers. The parietotemporal cortex is discussed in terms of its role in reading acquisition, with the left angular gyrus playing a key role. It is proposed that the functioning of the cortical network underlying reading is dependent on a combination of interacting factors, including physiological maturation, neural integrity, skill level, and the nature of the task.
Keywords: dyslexia, fMRI, maturation, reading ability
Introduction
The central aim of this study was to examine brain activation during visual sentence comprehension among poor readers. This subject is of interest because it is not yet known how poor readers' brains respond when comprehending text. This issue was explored among children with reading problems of varying degrees of severity, enabling us to address the question of continuity in the relationship between poor reading ability and brain function. The study also investigated whether any aberrant brain activation among poor readers changes with age. In normal readers, reading and reading-related skills continuously change in a dynamic manner throughout development (Wagner et al. 1997; Ehri 1999). Thus, developmentally driven physiological changes, such as increased left lateralization for language, may contribute to a different neural manifestation of reading problems at different ages. To examine this issue, the study assessed the brain activity of poor readers in the third and the fifth grades, in comparison to good readers.
Most studies investigating the neurobiological basis of reading difficulty have concentrated on lower-level tasks involving letters and words (e.g., Paulesu et al. 1996; Rumsey et al. 1997; Shaywitz et al. 1998, 2002, 2003, 2004; Brunswick et al. 1999; Georgiewa et al. 1999; Pugh 2000; Simos et al. 2000, 2002; Corina et al. 2001; Temple et al. 2001, 2003; Aylward et al. 2003; Eden et al. 2004). Because impaired phonological processing ability is thought to be the main source of reading difficulty (see Vellutino et al. 2004 for a review), much of the prior neuroimaging research has focused on word-reading tasks that vary in the degree of phonological processing that they require. To our knowledge, this is the first major fMRI study to investigate brain functioning among struggling readers during a higher-level reading comprehension task (Seki et al. 2001 report a small-scale study of Japanese dyslexic children reading kana sentences). How poor readers' brains respond when processing text, which requires more complex syntactic and semantic processing, may provide new information about the functioning of the impaired phonological processes when they are part of a more complex comprehension process.
One of the most consistent results in previous neuroimaging studies on dyslexic individuals using lower-level linguistic tasks such as decoding letters and words is a finding of reduced or absent activation in the left parietotemporal and occipitotemporal cortices (see Shaywitz SE and Shaywitz BA 2005 for a review). Underactivation of these regions has been associated with poor word recognition ability, which is the most common cause of reading difficulty. Inadequate word recognition appears to be the outcome of a basic problem in phonological processing. This seems to be the case independently of factors such as general intelligence and socioeconomic disadvantage (Vellutino et al. 2004). Deficits in phonological processing are expressed as problems in identifying, representing, and manipulating basic speech sounds, which in turn lead to difficulty in mapping sounds onto letters and acquiring letter-sound correspondences. The parietotemporal region (including the posterior aspects of the superior and middle temporal gyri, the supramarginal gyrus, and the angular gyrus) is thought to support various types of phonological processing involved in reading. By contrast, a more inferior occipitotemporal region is thought to be involved when word recognition skills become more automatic and direct visual access to the mental lexicon is the predominant reading strategy (Pugh et al. 2000; Shaywitz et al. 2002).
Like any cognitive task, reading is neurally underpinned not by a single area but a set of areas functioning as a large-scale cortical network. The neural signature of reading impairment is marked not only by reduced parietotemporal and occipitotemporal activation but also by increased left prefrontal activation (often attributed to compensatory covert articulatory processing, e.g., Pugh et al. 2000; Shaywitz et al. 2002). This study additionally examined the activation in these prefrontal components of the cortical network. In the discussion of the new findings, the results on sentence comprehension are integrated with previous work on impaired word reading.
Methods
Participants
The sample included third and fifth grade good and poor readers from public schools surrounding Pittsburgh in Allegheny County, Pennsylvania. The poor readers were participants in the Power4Kids Reading Initiative, a randomized trial, field study of remedial instruction for children with a wide range of reading difficulties. (For a full description see Torgesen et al. 2006, at http://www.ed.gov/rschstat/eval/disadv/title1interimreport/index.html) Criteria for inclusion in the project were a score at or below the 30th percentile on the combination of the Sight Word Efficiency and Phonological Decoding Efficiency subtests of the TOWRE during its initial administration (Test of Word Reading Efficiency; Torgesen et al. 1999) and a score at or above the fifth percentile on the Peabody Picture Vocabulary Test (PPVT: Dunn LM and Dunn LM 1997). Good readers (designated as average to above average by their teachers) were recruited for the fMRI study from the same schools. When the baseline measures were obtained, poor readers obtained significantly lower scores than good readers on the TOWRE, as shown in Table 1. There was some test-retest score variation on the 2 versions of the TOWRE administered at screening and baseline, with the baseline version used in the analyses. This choice, although resulting in some score overlap between good and poor readers, did not impact the results.
Table 1.
Demographic and behavioral profile of good and poor readers: fifth and third grades
| Group | Fifth grade | Third grade | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Good readers | Poor readers | Good readers | Poor readers | |||||||
| Sample size (n) | 12 (9 girls) | 23 (18 girls) | 14 (9 girls) | 18 (10 girls) | ||||||
| Measure | Mean | SD | Mean | SD | t(33) | Mean | SD | Mean | SD | t(30) |
| Note: Standard measures = standard scores for grade. SD, standard deviation. | ||||||||||
| Age (years) | 10.77 | 0.41 | 10.77 | 0.56 | 0.99 | 8.99 | 0.25 | 8.72 | 0.33 | 2.52* |
| Standard measures | ||||||||||
| TOWRE | 100.92 | 10.22 | 77.69 | 10.08 | 6.44*** | 118.07 | 11.17 | 89.67 | 6.30 | 9.11*** |
| PPVT | 117.08 | 9.73 | 95.32 | 11.89 | 5.40*** | 106.00 | 12.37 | 98.78 | 14.91 | 1.46 |
| Experimental task | ||||||||||
| Accuracy (% correct) | 0.98 | 0.02 | 0.95 | 0.07 | 1.72 | 0.97 | 0.03 | 0.91 | 0.06 | 3.31** |
| Response time (msec) | 3157 | 560 | 4208 | 885 | 3.73*** | 3520 | 421 | 5414 | 329 | 7.14*** |
P < 0.05.
P < 0.01.
P < 0.001.
The fifth grade sample consisted of 23 poor readers and 12 good readers. The third grade sample consisted of 18 poor and 14 good readers. This paper reports on the children's brain activity only at the time preceding remedial instruction. These children later participated in the postinstruction evaluation, thereby enabling a direct comparison of the same children in the 2 phases (in a treatment assessment study whose analysis is still in progress). The participants were all right-handed, native English speakers, with normal vision and hearing. Children were excluded from the study if they had brain injury, sensory disorders, psychiatric disorders, or attention deficit disorder, were on medication, had any metal in their bodies, or were claustrophobic. Of an initial sample of 121 scanned children, 54 were subsequently excluded from the final analysis for one or more of the following reasons: accuracy scores below 75% on the experimental task or inability to read well enough to perform the task (5), excessive head motion (more than 3 mm) (14), did not remain in the sample at posttest (24), and/or did not receive remedial instruction (10). One child was diagnosed with autism postscan.
Recruitment
Parents received explanatory materials about the reading project in the mail, including the fMRI study, and those expressing interest in the fMRI study were recruited. The children gave verbal informed consent in the presence of a parent or guardian, who gave signed informed consent. The children were paid for their participation. A parent questionnaire was used to verify that all participants met inclusion criteria. All protocols were approved by the University of Pittsburgh and Carnegie Mellon University Institutional Review Boards. Following recruitment and screening, the good and poor readers were scanned and baseline measures were administered.
Experimental Paradigm and Procedure
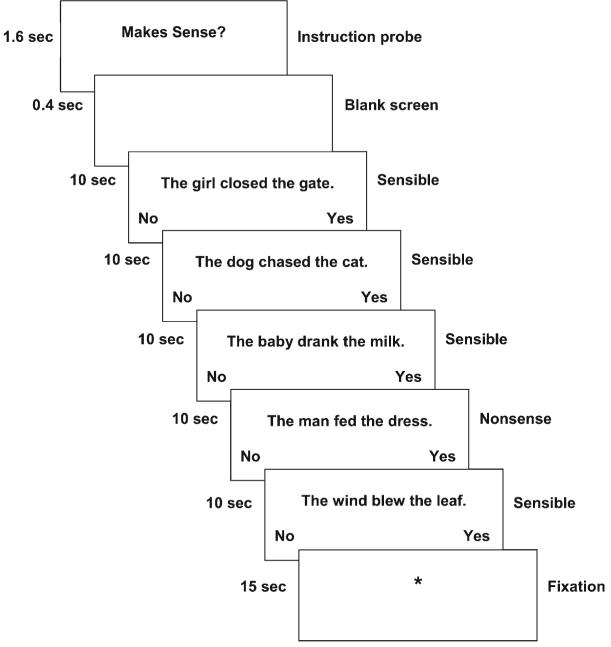
In the sentence comprehension task, the children decided whether a sentence they read made sense or not, as shown in Figure 1. This task was designed to be well within the reading ability of the poorest readers on the basis of a pilot study, to minimize performance-related confounds by assuring high accuracy rates. A sensibility (nonsense vs. sensible) × syntactic complexity (active vs. passive) blocked design was used. For the purposes of the present analyses, the 4 experimental conditions were combined to obtain a general effect of sentence processing. The data acquisition was split into consecutive runs to reduce the length of time children had to remain still while they concentrated on the task. Each run consisted of 4 stimulus blocks, one of each type. Five fixation blocks of 15 s each were interleaved with the 4 stimulus blocks to provide a control baseline comparison. The fixation consisted of a plus sign (+) centered on the screen. The probe “Makes Sense?” appeared at the beginning of each block. The probe was presented for 1.6 s followed by a 400-ms blank screen. Each block contained 5 stimulus sentences, one of which was a randomly placed sense-judgment distracter. Each sentence trial was 10 s in length: the sentence itself was presented in the middle of the screen for 9.5 s, followed by a 500-ms blank screen. An asterisk appeared at 8 s into the trial below the sentence to cue the participant if they had not yet responded. Participants used a right-hand button press to indicate “sensible,” and a left-hand press for “not sensible.” The words “no” and “yes” appeared at the bottom left and right corners of the screen as a reminder of the hand-to-response mapping. Two practice sentences, not included in the data analysis, preceded each acquisition. Head motion in the scanner was constrained using foam padding and surgical tape across the forehead.
Figure 1.
Diagram of a sentence block.
Prior to entering the scanner, the participants were trained on 2 sets of practice stimuli in order to introduce them to the experimental task and setting. The first set was practiced on a computer in order to acquaint the children with the task. The second set was practiced inside a full-scale scanner simulator in order to familiarize the children with the scanner environment. Head stability training was also part of the simulation.
fMRI Procedures
The data were collected using a Siemens Allegra 3.0T scanner with a commercial birdcage, quadrature-drive radiofrequency head coil. Data acquisition was conducted at the Brain Imaging Research Center of the University of Pittsburgh Medical Center, jointly established by Carnegie Mellon University and the University of Pittsburgh. The study was performed with a gradient-echo planar pulse sequence with time repetition = 1000 ms, time echo = 30 ms, and a 60° flip angle. Sixteen oblique-axial slices were imaged, and each slice was 5 mm thick with a gap of 1 mm between slices. The oblique-axial slices were positioned so that the most inferior slice was above the orbits anteriorly and passed through the fourth ventricle posteriorly. This resulted in nearly complete coverage of the cortex for most participants, with only small regions of orbitofrontal cortex and the inferior portions of the temporal poles falling outside the acquisition volume. The acquisition matrix was 64 × 64 with 3.125 × 3.125 × 5 mm voxels.
fMRI Analyses
To assess cortical activation in the participating groups, the data were analyzed using SPM99 (Wellcome Department of Cognitive Neurology). Images were corrected for slice acquisition timing, motion corrected, and normalized to the Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm voxels, and smoothed with an 8-mm Gaussian kernel to decrease spatial noise. High-pass filtering and global scaling were performed on each participant's data. Statistical analysis was conducted on individual and group data by using the general linear model as implemented in SPM99 (Friston et al. 1995). For each participant, the paradigm was modeled as a boxcar convolved with the standard SPM99 hemodynamic response function estimate, and contrast images were generated reflecting the difference between the mean of the parameter estimates for sentence reading with that for the fixation baseline.
Whole-brain multiple regression was used to examine the relationship between out-of-magnet reading ability and cortical activation during sentence processing across the entire sample of children. In these analyses, the dependent measure at each voxel was the participant's first-level contrast value for the difference between sentence reading and fixation, and the independent variables included the participant's raw TOWRE score (sum of Sight Word Efficiency and Phonemic Decoding Efficiency subtest scores), chronological age, raw PPVT score, mean response time in the sentence reading task, and gender. Vocabulary, response time, and gender were included as covariates in order to partial out any variation among the children arising from these factors that might impact the main effects of age and reading ability and the age by reading ability interaction. An initial model was run that included, in addition to the above covariates, the product of each participant's age and TOWRE score in the regression in order to identify voxels where there was an interaction between these variables. A reduced model including all covariates but excluding this interaction term was then run on the remaining voxels in order to examine main effects of age and TOWRE in voxels that did not show a reliable interaction between these variables. The slope of the regression relating the raw TOWRE scores to brain activation was calculated at each voxel, and the t-map testing the difference of these parameter estimates from zero was thresholded at P < 0.0005, uncorrected, extent threshold = 10 voxels. This probability map therefore shows areas where there was a reliable partial correlation between reading ability and activation after controlling for the other variables in the model. Similar probability maps were generated for the partial correlation of age with activation and for the interaction of the 2 variables. The probability maps were superimposed on the high-resolution normalized T1-weighted individual template image for viewing. Labels for coordinates of activation were confirmed in MNI space (Tzourio-Mazoyer et al. 2002) and the Talaraich Daemon (Lancaster et al. 2000) as implemented in AFNI (Cox 1996).
To explore the effect of reading ability on brain activation in more detail, secondary ROI-based regression analyses were performed using anatomically defined regions of interest (Tzourio-Mazoyer et al. 2002). The ROIs were selected on the basis of the clusters showing significant effects in the whole-brain voxelwise analyses but included all voxels in the anatomically defined regions. These analyses provided a way to examine the gradient of the regression slope and to evaluate the dispersion of individual scores around the regression line. In these analyses, the contrasts of parameter estimates (reading minus fixation) were first extracted from each participant's first-level general linear model across all voxels in each ROI. These contrast values were averaged over the voxels in the ROI for each participant and then entered as dependent variables in the regression models including all participants, with age, PPVT, response time, and gender included as covariates.
Results
Behavioral Data
Comprehension accuracy was over 90% in all 4 groups of children (good and poor readers at both ages), indicating that they were semantically processing the sentences. The response accuracies did not reliably differ between good and poor readers in the fifth grade, but in the third grade, the good readers were reliably more accurate, as shown in Table 1. In both grades, poor readers were significantly slower at responding than good readers.
fMRI Data
Reading Ability and Brain Activation
The results of the whole-brain multiple regression analyses (controlling for chronological age, PPVT, response time, and gender) revealed an increasing linear relation between brain activation and reading ability in several parietotemporal regions, as indicated in Table 2 and Figure 2a. Participants with lower reading scores had less activation in the left middle temporal gyrus (Wernicke's area: BA 22), the right inferior parietal lobule (BA 40), and the left postcentral gyrus (BA 2). There were no areas in which poor readers exhibited greater activation than good readers, as indicated by the absence of significant negative partial correlations between reading ability and brain activation.
Table 2.
Multiple regressions: voxel clusters showing significant partial correlations
| Cortical region | BA | Cluster size | Peak t-value | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Note: The threshold for significant activation was P < 0.0005 for a spatial extent of 10 voxels, uncorrected for multiple comparisons. Region labels apply to the entire extent of the cluster, with peak maxima designated by first locale cited. The t-values and MNI coordinates are for the peak activated voxels in each cluster. | ||||||
| Main effects | ||||||
| Reading ability (positive) | ||||||
| Left middle temporal | 22 | 13 | 4.24 | −64 | −50 | 8 |
| Left postcentral | 2 | 17 | 4.10 | −64 | −12 | 24 |
| Right inferior parietal, supramarginal | 40 | 41 | 4.25 | 62 | −50 | 38 |
| Age (negative) | ||||||
| Right inferior frontal (pars triangularis) | 45 | 35 | 4.00 | 44 | 18 | 20 |
| Right middle frontal | 9 | 12 | 3.88 | 20 | 44 | 38 |
| Interaction | ||||||
| Age × reading ability | ||||||
| Left angular | 39 | 64 | 4.43 | −36 | −66 | 32 |
| Right inferior frontal (pars triangularis) | 45 | 112 | 4.29 | 46 | 24 | 26 |
Figure 2.
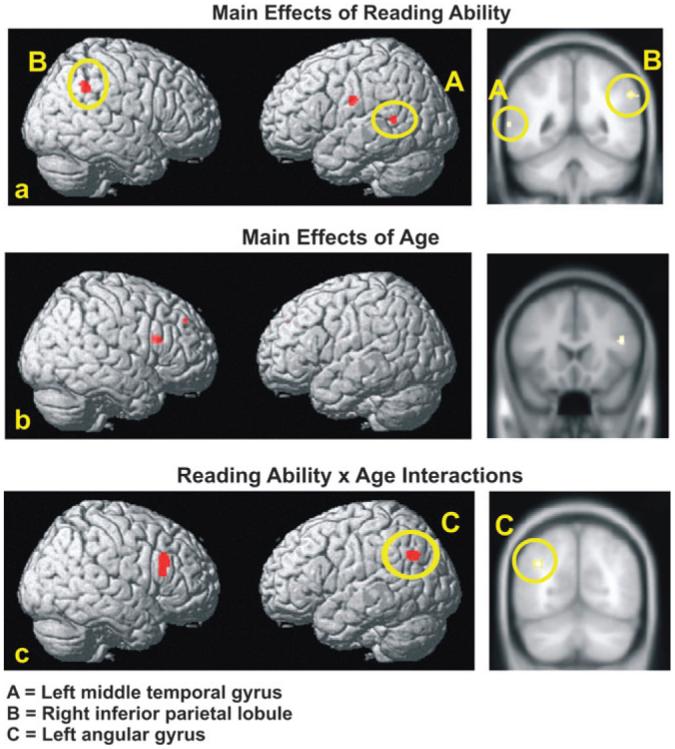
Multiple regression analyses: Main effects of reading ability, age, and age × reading ability interaction. Top row (panel a) presents surface rendering and canonical view of regions correlated with reading ability. Middle row (panel b) presents surface rendering and canonical view of regions correlated with age. Bottom row (panel c) shows surface rendering of regions and canonical view showing age × reading ability interaction. Yellow ovals encircle parietotemporal activation.
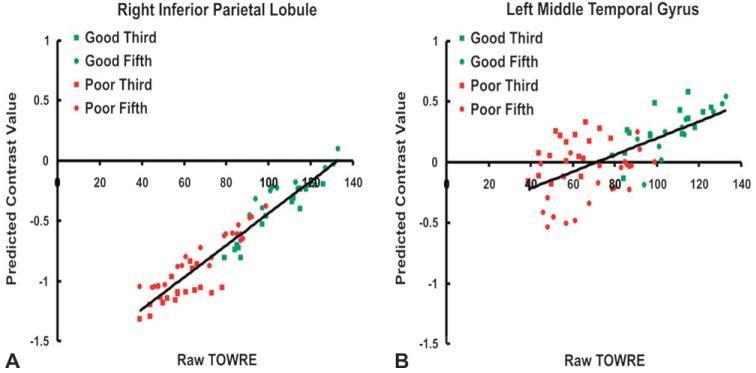
Additional ROI-based analyses (described in the Methods) examined the gradient of the regression slope and evaluated the dispersion of individual scores around the regression line in the left middle temporal gyrus, the right inferior parietal lobule, and the left postcentral gyrus. The results of this analysis showed that the activation in all 3 of these areas increased approximately linearly with reading score, as shown in Figure 3, which plots the mean predicted contrast value as a function of reading score for each participant from the full regression model for the left middle temporal gyrus and the right inferior parietal lobule. As shown in the scatter plots of Figure 3, the good readers (green symbols) and poor readers (red symbols) fall along the same straight line that relates reading ability to activation. Scatter around the regression line was greater in the left middle temporal gyrus among poor readers, with fifth grade children tending to activate less than third grade children at a given reading level.
Figure 3.
Regression scatter plots depicting the main effects of reading ability (A) in the right inferior parietal lobule and (B) in the left middle temporal gyrus. Raw TOWRE scores reflect the sum of the Sight Word Efficiency and Phonemic Decoding Efficiency subtests.
The predicted contrast values for some of the key ROIs included values less than zero. It is not clear why these values or the intercept are negative, although it is evident from the slope that activation is linearly modulated by reading ability (as shown in Fig. 3). Although negative activation contrast values being correlated with an independent variable is not uncommon, the reasons for the negativity are not well understood (McKiernan et al. 2003). One plausible interpretation of the negativity is that some of the parietotemporal regions involved in reading are also involved in a default network that functions during the fixation condition. Regardless of the cause of the negative intercept, the linear slope indicates the systematic relation between activation during reading and the independent measure of reading ability.
Age and Brain Activation
To assess the influence of age on brain activation during reading, the relationship between chronological age and brain activation was examined with raw TOWRE scores, raw PPVT scores, response time, and gender entered as covariates in the multiple regression analysis. A negative partial correlation was found between age and activation in the right inferior frontal gyrus (pars triangularis: BA 45) and the right superior frontal gyrus (BA 9), as shown in Table 2 and Figure 2b. This finding indicates that the activation in these regions decreased with age, irrespective of reading ability. No positive partial correlations were found.
Age × Reading Ability Interactions
The full multiple regression model that included the interaction term for the participants' age and their raw TOWRE scores as an additional covariate revealed significant positive age by reading ability interactions in the left angular gyrus (BA 39) and the right inferior frontal gyrus (pars triangularis: BA 44), as indicated in Table 2 and Figure 2c. To determine what form this interaction takes, separate ROI-based regressions were performed for good and poor readers, which examined age effects within each group. This analysis focused on the left angular gyrus because right pars triangularis showed an additional effect of age. Among good readers, the results indicated that older children showed more activation than younger children (F1,21 = 3.39, P = 0.08). Although marginally significant, this result points to a positive linear trend with age. Among poor readers, older children showed a slight and nonsignificant trend toward less activation than younger children (F1,36 = 1.29, P = 0.26). Accordingly, the significant age × reading ability interaction may be attributed to a widening gap between good and poor readers with age. This widening gap appears to arise primarily from increased activation among good readers with age.
In sum, the results of the multiple regression analyses indicate that poorer reading ability is associated with less parietotemporal activation in the left middle temporal gyrus, the right inferior parietal lobule, and the left postcentral gyrus. An additional parietotemporal region, the left angular gyrus, showed an interaction between age and reading ability, as did a region of the right inferior frontal gyrus. Age made an independent contribution to variation in brain activation, with younger children showing more right inferior frontal activation. These findings indicate that there is a systematic relationship between brain activation and reading ability. In some cortical regions, this relationship changes with age.
Discussion
This first investigation of brain activity during sentence comprehension in children who are struggling readers offers new insight into the neural bases of reading difficulty and determines some facets of its developmental time course. The results revealed an atypical brain response among poor readers when they were comprehending sentences, as well as evidence of a developmental trend between third and fifth grade.
A central finding was that poor reading ability was associated with reduced activation relative to good readers bilaterally in the parietotemporal cortex. The form of this relationship was regionally dependent. A positive linear relationship between reading ability and cortical activation was found in the superior aspect of the left middle temporal gyrus (Wernicke's area), the right inferior parietal lobule, and the left postcentral gyrus. These findings indicate a continuous, linear relation between reading ability and activation in these regions. Accordingly, less activation occurred when reading difficulties were more acute. This result shows that good and poor readers lie along the same continuum in the reading-related processes supported by these regions.
In another parietotemporal region, namely, the left angular gyrus, the degree of underactivation observed among poor readers during sentence processing was greater in the older age group. Thus, the relationship between reading ability and brain activation was additionally influenced by age. The findings in the older age group are consonant with previous studies at the letter and word levels that show less activation among poor readers in the left angular gyrus (Shaywitz et al. 1998; Pugh et al. 2000; Temple et al. 2001). Although a cohort effect cannot be ruled out in the context of a cross-sectional study, the current findings suggest that activation patterns among good and poor readers in this region may become increasingly divergent over time. The major contributing factor to this trend appears to be the gradual increase in activation among good readers with age. These results may signify that in certain components of the language network, different developmental trajectories distinguish good and poor readers.
Age appears to exert an independent effect on cortical activation during sentence comprehension. This effect emerged in the right hemisphere (pars triangularis and superior frontal gyrus) and followed a decreasing linear trend, such that activation in these regions lessened with age. This finding suggests that with biological maturation and/or other environmental influences such as increasing linguistic or scholastic experience, the recruitment of these right hemisphere regions is reduced. In a spatially adjacent region of right pars triangularis, an interaction between age and reading ability was found, following a similar trend to that observed in the left angular gyrus. Together, the changes in right pars triangularis could reflect the advent of regional specialization among normally progressing readers, which fails to occur among poor readers. Although the specific role played by the right inferior frontal gyrus during sentence reading is uncertain, it could involve aspects of phonological and semantic processing (Booth et al. 2003, 2004).
Parietotemporal Cortex: A Central Player in Reading Acquisition
Previous research examining the neurobiological underpinnings of reading difficulty has focused on language processing at the level of letters and words, particularly phonological processing. Poor phonological processing is viewed as a key factor in the failure to develop adequate word recognition skills, representing a primary impediment to the acquisition of grapheme-phoneme knowledge. Although both the inferior frontal and parietotemporal regions are engaged during phonological processing, the parietotemporal cortex is thought to be critical to analyzing the written word (e.g., transforming the orthographic representation into the underlying linguistic structures: Shaywitz et al. 2002; Booth et al. 2003). Our new findings on sentence comprehension are largely compatible with previous brain imaging studies of dyslexic children performing single word-reading tasks, which consistently show abnormal parietotemporal activation among dyslexic readers (see Shaywitz SE and Shaywitz BA 2003, 2005 for discussion). The occurrence of a similar pattern of under-activation in a sentence comprehension task strongly suggests that this area continues to be a discriminating factor in the development of reading ability beyond the level of reading single words.
In the case of sentence reading, parietotemporal under-activation among poor readers could be related to problems associated with phonological working memory functions at different levels of analysis: from mapping print to sound at the level of words, to the temporary storage and integration of sound-based word information at the sentence level. Because sentence reading also involves syntactic and semantic processes, it is plausible that the parietotemporal region may be involved in these facets of reading as well, possibly functioning as a storage medium for integrating multiple elements of linguistic information in verbal working memory. In addition, the fact that the sentences were highly imageable (e.g., the man drove the banana) suggests that the degree to which linguistic stimuli elicit visual imagery may also contribute to activation in this region (Just et al. 2004).
Both the left and the right parietotemporal regions have been linked to verbal working memory processes during reading comprehension, although the extent to which each hemisphere is recruited is influenced by task demands (Keller et al. 2003; Xu et al. 2005) and individual differences (Grossman et al. 2002). Despite some functional overlap, it is likely that the 2 homologues subserve slightly different cognitive processes in reading comprehension. Functional overlap and functional specialization may likewise characterize the anatomical structures within a particular brain region. Consequently, different cortical structures may operate in concert to perform a particular cognitive task such as reading but may vary in the manner in which each contributes to task performance. For instance, Wernicke's area is strongly linked to language comprehension and may play a central role in sustaining semantic access during reading. An adjacent structure, namely, the left angular gyrus, is thought to be pivotal in the mapping between phonological and orthographic representations of words (Shaywitz et al. 2002; Booth et al. 2003, 2004), as well as for the integration of these word forms with their semantic representations (Booth et al. 2003).
Developmental Trends
In the present study, the diverging patterns of parietotemporal activation among good and poor readers with increasing age were centered in the left angular gyrus, supporting the idea that this structure is critically involved in the normal process of learning the mappings among different representations of words. Other work has also pointed to the left angular gyrus as playing a crucial role in the development of skilled reading (Pugh et al. 2000; Booth et al. 2004). Booth et al. (2004), for instance, found significantly greater activation in the left angular gyrus among adults as compared with children when performing tasks requiring conversion between phonology and orthography (visual rhyming and auditory spelling). The current results suggest that the disparity between good and poor readers in the consolidation of word mappings was greater in the fifth grade than in the third grade, presumably due to the age-related increases in this ability among normally progressing readers. These age-related increases could reflect the gradual emergence of regional specialization. That is, as learning occurs, a process of neural fine-tuning may transpire in which specific language functions become progressively localized to the cortical areas that are most proficient at meeting the cognitive demands of the task. As a result, processing may become more efficient. Prior research using different cognitive tasks supports this claim (e.g., Gaillard et al. 2000, 2003; Booth et al. 2001; Holland et al. 2001; Johnson 2001; Johnson et al. 2002; Sachs and Gaillard 2003; Turkeltaub et al. 2003; Brown et al. 2005).
Conclusions and Implications
These new findings have both practical and theoretical implications. An important result is that the regions found to be underactivated among poor readers during sentence comprehension are largely consistent with those found to be underactivated in studies of word-level processing. The parietotemporal area, particularly in the left hemisphere, appears to be a key locus of dysfunction in children who experience difficulty in learning to read. This conclusion is further supported by the finding that parietotemporal under-functioning typified struggling readers across a wide range of performance, manifesting in children with more severe reading impairment as well as those with less acute reading problems. Interestingly, this pattern of brain activation was found despite poor readers' relatively high accuracy scores on the experimental task, providing some indication that although these children may have deficits in phonological processing (a lower-order function), higher-order comprehension processes may still function adequately.
The results also point to the importance of assessing reading problems within a neurodevelopmental context. A potentially significant discovery is that neural function during sentence reading was influenced by skill level as well as age, with the 2 factors contributing both independent and interacting effects. A critical implication of this result is that the age at which poor readers are examined may influence the expression of impaired cortical function. We posit that the functioning of the cortical network involved in reading is dependent on a combination of interacting factors, including skill level, the maturity and integrity of the underlying neural systems, and the nature of the task. Various components of this network may be differentially called into play as children develop, gain increasing experience with text, and establish more efficient reading strategies. According to this position, a dynamic approach to the assessment of reading problems, which takes into account the normally developing functional organization of the neural systems involved in reading, may prove productive. Data acquired at different stages of reading development have the potential to enhance our knowledge of the relationship between changes in brain function and the effects of age and training, providing a new source of information that can inform models of reading development and impairment.
Notes
This research was supported by grants from the R.K. Mellon Foundation, the National Institute of Mental Health (Grant MH029617), and the William and Flora Hewlett Foundation. Participants were recruited through the Power4Kids program, which is a public-private partnership including the Haan Foundation for Children; Institute of Education Sciences, US Department of Education; Heinz Endowments; Smith Richardson Foundation; W.K. Kellogg Foundation; Grable Foundation; Rockefeller Foundation; Ambrose Monell Foundation; Raymond Foundation; and Barksdale Reading Institute. For a full description of this project, see http://www.ed.gov/rschstat/eval/disadv/title1interimreport/index.html We thank Cindy Haan and Joe Torgesen for leadership of the Power4Kids program. We also thank Donna Durno, Rosanne Javorsky, and the Allegheny Intermediate Unit for their central coordinating efforts throughout the project.
References
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, Richards AL, Thompson JB, Cramer SC. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. J Cogn Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Choy J, Gitelman DR, Parrish TB, Mesulam MM. Modality-specific and -independent developmental differences in the neural substrate for lexical processing. J Neurolinguistics. 2003;16:383–405. [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TB, Mesulam MM. The development of specialized brain systems in reading and oral language. Child Neuropsychol. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlagger BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCroy E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Corina DP, Richards TL, Serafini S, Richards AL, Steury K, Abbott RD, Echelard DR, Maravilla KR, Berninger VW. fMRI auditory language differences between dyslexic and able reading children. Neuroreport. 2001;12:1195–1201. doi: 10.1097/00001756-200105080-00029. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test—revised. American Guidance Service; Circle Pines (MN): 1997. [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NAE, Agnew JA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Ehri LC. Phases of development in learning to read words. In: Oakhill J, Beard R, editors. Reading development and the teaching of reading: a psychological perspective. Blackwell Science; Oxford: 1999. pp. 79–108. [Google Scholar]
- Friston K, Ashburner J, Frith C, Poline J-B, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf J, Knab R, Glauche V, Kaiser WA, Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, Gee J. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strwasburg RH, Schmithorst VJ, Balls WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–943. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Halit H, Grice SJ, Karmiloff-Smith A. Neuroimaging of typical and atypical development: a perspective from multiple levels of analysis. Dev Psychopathol. 2002;14:521–536. doi: 10.1017/s0954579402003073. [DOI] [PubMed] [Google Scholar]
- Just MA, Newman SD, Keller TA, McEleney A, Carpenter PA. Imagery in sentence comprehension: an fMRI study. Neuroimage. 2004;21:112–124. doi: 10.1016/j.neuroimage.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. Brain imaging of tongue-twister sentence comprehension: twisting the tongue and the brain. Brain Lang. 2003;84:189–203. doi: 10.1016/s0093-934x(02)00506-0. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment Retard Dev Disabil Res Rev. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Arch Neurol. 1997;49:527–534. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Sachs BC, Gaillard WD. Organization of language networks in children: functional magnetic resonance imaging studies. Curr Neurol Neurosci Rep. 2003;3:157–162. doi: 10.1007/s11910-003-0068-z. [DOI] [PubMed] [Google Scholar]
- Seki A, Koeda T, Sugihara S, Kamba M, Hirata Y, Ogawa T, Takeshita K. A functional magnetic resonance imaging study during sentence reading in Japanese dyslexic children. Brain Dev. 2001;23:312–316. doi: 10.1016/s0387-7604(01)00228-5. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan JM, Marchione KE, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Neurobiological indices of dyslexia. In: Swanson HL, Harris KR, Graham S, editors. Handbook of learning disabilities. Guilford Press; New York: 2003. pp. 514–531. [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Biol Psychiatry. 2005;57:1301–1309. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, et al. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Menci WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: a magnetic source imaging approach. Cereb Cortex. 2000;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Berier JI, Foorman BR, Castillo EM, Davis RN, Fitzgerald M, Papanicolaou AC. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci USA. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuro-report. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Myers D, Schirm A, Stuart E, Vartivarian S, Mansfield W, Stancavage F, Durno D, Javorsky R, Haan C. Closing the reading gap: first year findings from a randomized trial of four reading interventions for striving readers. Volume II. National Assessment of Title I: Interim Report to Congress, Institute of Educational Sciences, National Center for Education Evaluation and Regional Assistance, US Department of Education [Internet] 2006. [cited 2007 February 16]. Available from: http://www.ed.gov/rschstat/eval/ disadv/title1interimreport/index.html. [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency (TOWRE) Pro-ed; Austin (TX): 1999. [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Wagner R, Torgesen JK, Rashotte CA, Hecht SA, Barker TA, Burgess SR, Donahue J, Garon T. Changing relations between phonological processing abilities and word-level reading as children develop from beginning to skilled readers: a 5-year longitudinal study. Dev Psychol. 1997;33:468–479. doi: 10.1037//0012-1649.33.3.468. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]