Abstract
Background
Propofol may produce amnesia by affecting encoding. The hypothesis that propofol weakens encoding was tested by measuring regional cerebral blood flow during verbal encoding.
Methods
17 volunteer participants (12 M, 30.4±6.5 years old) had regional cerebral blood flow measured using H2O15 positron emission tomography during complex and simple encoding tasks (deep vs. shallow level of processing), to identify a region of interest in the left inferior prefrontal cortex (LIPFC). The effect of either propofol (n=6, 0.9 mcg/ml target concentration), placebo with a divided attention task (n=5), or thiopental at sedative doses (n=6, 3 mcg/ml) on regional cerebral blood flow activation in the LIPFC was tested. The divided attention task was expected to decrease activation in the LIPFC.
Results
Propofol did not impair encoding performance or reaction times, but impaired recognition memory of deeply encoded words 4 hours later (median recognition of 35% (17–54 interquartile) of words presented during propofol versus 65% (38–91) before drug, p<0.05). Statistical parametric mapping analysis identified a region of interest of 6.6 cu.cm. in the LIPFC (T=7.44, p=0.014). Regional cerebral blood flow response to deep encoding was present in this region of interest in each group before drug (T>4.41, p<0.04). During drug infusion only the propofol group continued to have borderline significant activation in this region (T=4.00, p=0.063).
Conclusions
If the amnesic effect of propofol were solely due to effects on encoding, then activation in LIPFC should be minimal. As LIPFC activation was not totally eliminated by propofol, the amnesic action of propofol must be present in other brain regions and/or affect other memory processes.
INTRODUCTION
One of the still unsolved mysteries of anesthesia is how temporary amnesia is produced during wakefulness by drugs such as midazolam or propofol. Dense amnesia even for intense events such as intraoperative wake-up has been demonstrated for propofol. 1 The memories most sensitive to propofol are those that are consciously accessible in rich detail and include contextual information such as when and where they occurred. 2,3 These memories are encoded and retrieved by the episodic memory system, which includes the hippocampus as the central, key component of memory processing. 4 Episodic memory can be conceptualized as the processes of encoding, storing and then retrieving information. Though testing recognition memory some time after the administration of propofol clearly demonstrates the presence of amnesia, one cannot determine which memory process or processes are affected. 2 Imaging brain activity using electroencephalography, positron emission tomography (PET) or functional magnetic resonance imaging during behavioral paradigms that isolate specific memory processes can characterize the brain processes underlying specific components of episodic memory function. 5–7 These neuroimaging techniques may reveal differences in the processes and neural structures underlying a given behavior (e.g. encoding) in the setting of matched behavioral performance, as has been done in the elderly versus the young. 8,9 Specifically, at similar performance levels the elderly recruit additional brain regions during encoding, and do not demonstrate as large event related potentials in the left prefrontal cortex as the young during semantic retrieval and encoding operations. In other words, behavioral measures alone are insufficient to fully characterize memory processes.10,11 The current study focuses on the processes and neural structures that underlie encoding, using PET imaging of cerebral blood flow to more fully characterize the effects of propofol, if any, on this key memory process.
Encoding is defined as the process of acquiring and representing information in memory. For the purposes of the current study memory processes after encoding are divided into working and long-term memories. Encoding is closely related to working memory, and overlapping brain regions are involved. 12–15 Working memory is defined as a short term retention of information no longer available in the environment, and is of limited capacity (e.g. 7 ± 2 items) 12,16 After approximately 18 seconds information can only be retained in working memory by ongoing rehearsal. 17 Long term memory is defined here as memory that is not working memory, i.e. memory retained longer than 20 seconds after encoding in the absence of rehearsal. There are a number of phases of long-term memory, which we do not differentiate for the purposes of the current study. 18
One method that allows assessment of the effects of a drug on working and long-term memory processes as defined above is the continuous recognition task. We previously showed that at low doses propofol did not impair encoding or working memory as measured by behavioral responses on the continuous recognition task, but still produced amnesia at the end of the study day. 19 Despite normal encoding performance by behavioral measures in the presence of propofol, the processes and neural structures underlying this behavior might still be affected by propofol. We have recently presented suggestive evidence that event related potential measures of recognition memory in the presence of propofol are altered even when performance is unchanged. 20 Similar changes by propofol of brain processes underlying encoding could potentially affect longevity of memories over time. Such subtle effects on verbal memory, for instance, have been demonstrated using transcranial magnetic stimulation. 21,22
One brain region important in verbal encoding is the left inferior pre-frontal cortex (LIPFC). A behavioral paradigm that isolates encoding activity and reveals this brain region as important in this aspect of episodic memory is the levels of processing manipulation (LoP). This task involves evaluations of a series of words using either semantic, i.e. meaning based, or stimulus based processing. 23–25 In this task words could be categorized as representing a living object, or whether the word was spoken in a male or female voice, for example. Semantic evaluations invoke more complex memory operations that contact already stored knowledge. These operations result in deeply encoded memories that are enhanced and better retained than those formed during shallow, stimulus based processing. Memory processes underlying encoding are isolated by this task as evaluation occurs on the first presentation of the stimulus. These are revealed by the comparison of brain images obtained during deep versus shallow categorizations. The LIPFC and, in some studies, the hippocampus are identified using this methodology. 8,24,26 As the LoP improvement in memory is preserved in the presence of benzodiazepines, albeit at a lower level, this manipulation can be used to index encoding processes in the presence of doses of drug that produce memory impairment.27,28
As the dose of propofol increases, associated sedation occurs. 2 The difference between an amnesic dose of propofol without and one with associated sedation is small and not predictable in advance in a given individual. Behavioral measures reveal that higher, sedative doses of propofol can interfere with working memory and thereby impair encoding. 19 Thus, in the current study it is important to assess the effects of attention and sedation on the brain processes underlying encoding in order to clearly interpret the effects of propofol on these.
A behavioral manipulation that influences encoding via attentional mechanisms is the divided attention task. This task requires the participant to pay attention to a diversionary task when an encoding task is performed. The need for divided attention during encoding decreases subsequent recall or recognition memory. 29 Importantly for the current study, impaired encoding from divided attention is associated with decreased brain activation. 30–34 Thus, this task was included in the placebo group in the current study to isolate attentional effects in the LIPFC during encoding. Likewise, to assess the influence of sedation on LIPFC responses to the levels of processing manipulation, thiopental is included as an additional control group, as it interferes with memory principally by sedation. 2
The hypothesis tested in this study is that amnesic doses of propofol weaken encoding, as measured by effects on the rCBF response to the levels of processing manipulation in the LIPFC region of the prefrontal cortex. Our study is designed to closely parallel that of Kapur et al, who were able to isolate LIPFC activity using this paradigm. 24 A dose of propofol was chosen to produce amnesia but not sedation. 2,19,35 Behavioral measures of sedation were obtained by measuring accuracy of word categorizations and associated reaction times during the encoding tasks. Amnesic effects were assessed by recognition of words at various times after encoding, including at the end of the study day. The divided attention and thiopental groups served as controls for attentional and sedation effects on imaged brain responses. As explained below, fewer numbers of participants completed the study than anticipated. Thus, the primary response measure to address the hypothesis is the effect size of brain activation in the LIPFC, as estimated by T values of deep versus shallow encoding brain image contrasts in the study groups.
MATERIAL AND METHODS
Participants
Twenty-five healthy normal volunteers (19 M) were recruited through newspaper advertisements and paid for their participation. Participants were right-handed, proficient English speakers with normal hearing between 18 and 45 years of age (mean age 31.0 ± 10.5). Exclusion criteria included use of psychoactive medication, history of recreational drug abuse, head trauma resulting in loss of consciousness, psychiatric, neurologic, cardiovascular, or respiratory disease, major psychiatric disease in a first degree relative, claustrophobia, carpal tunnel syndrome, allergy to eggs, or acute intermittent porphyria. This investigation was approved by the Hospital Institutional Review Board and Radiation Safety Committees of Memorial Sloan-Kettering Cancer Center and Weill Cornell Medical Center, New York, New York. Informed consent was obtained in writing before accrual of participants took place.
Procedures
Behavioral Tasks
The levels-of-processing paradigm was used to encode auditory stimuli into verbal memory. In the deep encoding task, participants performed a semantic categorization task. Immediately after hearing each word, participants categorized it as representing either a living or a nonliving object. Choices were communicated to the investigators with a button press response with the right hand. Likewise, during shallow encoding participants categorized the voice speaking the word as male or female. Participants served as their own controls in the three study groups, those being propofol, placebo with a divided attention task (placebo/DA) or thiopental. The two study conditions for each group consisted of baseline observations followed by repetition of observations after study drug administration was begun using STANPUMP* computer controlled infusion.
Timeline
Before Study Day
During an orientation session detailed information was given on study procedures including radiation dose. Tests of handedness (Edinburgh Handedness Inventory 36) and vocabulary (vocabulary subtest of Wechsler Adult Intelligence Scale -Revised) were administered, followed by a brief physical examination. Participants were screened for normal hearing using the Coren-Hakstian Hearing Screening Inventory before recruitment into the study. 37 Informed consent was obtained at that time.
Study Day Procedures
Preparation
On the study day participants arrived about 8 am, being nil per os since midnight. A 64-channel electroencephalography cap (Neuroscan, El Paso, TX) was applied using standard procedures. Electroencephalography data are not reported here. Venous catheters were inserted before transfer to the PET suite. Dextrose 5% ½normal saline at approximately 100 cc • hr−1 was administered intravenously. All participants practiced the divided attention task and were informed that they might need to perform it after the start of drug infusion. However, they were not informed that the task was only used in the placebo group.
Behavioral tasks
Participants underwent a series of encoding and recognition tasks, with all stimuli presented as auditory words through earphones. Participants were instructed to keep their eyes open and fixated on a cross displayed on a projection mirror immediately above their head while in the PET scanner.
The current study focused on encoding tasks only, as accruals were not sufficient to image recognition-related brain activity (see statistical analysis section below). However, the behavioral results from the recognition tasks are presented as a measure of memory over the study day. The shallow encoding task always preceded the deep encoding task, in order to maintain a constant time interval between deep encoding and subsequent recognition tasks. Two recognition tasks were obtained following the encoding tasks in both the baseline and drug conditions. Each recognition task comprised two PET scans as explained below.
PET imaging in relation to behavioral tasks
The participant’s total time in the PET scanner was approximately 2.5–3 hours, with scans obtained every 12 minutes (see table 1). The interval was longer just before drug infusion was started, to allow for a brief break for the participant, and to allow establishment of the target drug concentration after the start of drug infusion.
TABLE 1.
Timeline of study
| 8:00 AM | Preparation: EEG cap applied, venous catheter inserted, saline administered intravenously, practice tasks. | ||
|---|---|---|---|
| PET scan # | Tasks | ||
| t=0 | 1 | Baseline Shallow Encoding | Is the word spoken by a male or female voice? |
| t+12 min | 2 | Baseline Deep Encoding | Does the word represent a living object? |
| t+24 min | 3 | Recognition 1 (Rcg 1, 18 min after baseline deep encoding) | Is the word old/or new? (from baseline deep encoding);
scan during either old/new |
| t+36 min | 4 | Is the word old/or new? (from baseline deep encoding);
scan during opposite old/new from scan 3 |
|
| BREAK | BREAK | Short Rest, reposition in scanner, CT Scan and alignment, Start drug infusion
(Propofol= 0.9 mcg/ml, Thiopental= 3 mcg/ml). |
|
| t+61 min | 5 drug | Recognition 2 (Rcg 2, 55 min after baseline deep encoding) | Is the word old/or new? (from baseline deep encoding);
scan during either old/new |
| t+73 min | 6 drug | Is the word old/or new? (from baseline deep encoding);
scan during opposite old/new from scan 5 |
|
| t+85 min | 7 drug | Drug Shallow Encoding | Is the word spoken by a male or female voice? |
| t+97 min | 8 drug | Drug Deep Encoding | Does the word represent a living object? |
| t+109 min | 9 drug | Recognition 1 (Rcg 1, 18 min after drug deep encoding) | Is the word old/or new? (from drug deep encoding);
scan during either old/new |
| t+121 min | 10 drug | Is the word old/or new? (from drug deep encoding);
scan during opposite old/new from scan 9 |
|
| t+133 min | 11 drug | Recognition 2 (Rcg 2, 42 min after drug deep encoding) | Is the word old/or new? (from drug deep encoding);
scan during either old/new |
| t+145 min | 12 drug | Is the word old/or new? (from drug deep encoding);
scan during opposite old/new from scan 11 |
|
| Out of scanner, rest 2.5 hours, lunch break, EEG/IV removal, ± MR structural scan | |||
| Time from deep encoding: 286 min (baseline),207 min (drug) | END OF DAY RECOGNITION : Is the word old/new (from baseline/drug shallow/deep encoding). | ||
The baseline condition consisted of scanning during shallow and deep encoding tasks. Following this, two recognition scans were needed to image the first recognition task of baseline words. The reason for needing two scans over one recognition task was as follows. One scan was obtained during presentation of old words presented during the previous deep encoding task, while the other was obtained during presentation of new words.† The order of old and new word scans was counterbalanced across participants. Each recognition scan was immediately preceded and followed by presentation of words of the opposite category than those presented during imaging, so that to the participants each recognition scan involved the same ratio of old and new words. Thus, two recognition scans comprise one recognition task for the full set of deeply encoded words in both baseline and drug conditions.
Drug infusion was started after the first recognition task, and then a second recognition task again comprising two PET scans of the baseline encoded words was obtained, in order to assess the effect of the presence of drug on recognition of words encoded in the absence of drug. After this second recognition task, shallow and deep encoding tasks/scans in the drug condition were obtained, followed by two more sets of recognition scans, to match the baseline condition. Drug infusion was then stopped.
End of Day Recognition task
Approximately 2.5 hours after PET scanning and drug infusion was finished, a series of four recognition tasks (end of day recognition, EODrcg) was given in randomized order, in which participants heard words previously presented (old words) during both shallow and deep encoding tasks in baseline and drug conditions, along with an equal number of distractor words (new words) not heard before. Participants made a yes/no decision regarding whether they had heard the word during PET scanning. Recognition tasks were administered at approximately 286 minutes after deep encoding in the baseline condition and approximately 207 minutes after deep encoding in the drug condition. Participants were discharged home after meeting standard criteria for discharge for ambulatory surgery.
Due to scheduling issues, 8 volunteers underwent structural magnetic resonance imaging scanning late in the afternoon in the same facility after lunch but before delayed recognition testing, while 9 returned for a magnetic resonance imaging scan on a separate occasion.
Materials
O-15 administration and PET brain images
For each PET scan approximately 10 mCi of H215O was delivered intravenously at a constant rate over 20 seconds via an infusion pump. Scans were obtained on a GE Advance scanner (GE Medical Systems, Waukesha, WI) in the 3D “septa out” mode. The resolution of the PET camera in this mode is approximately 5.2 mm in all dimensions. Imaging started with the arrival of radioactivity at the brain. Three 30-second frames were obtained during each scan, corresponding to the highest rate of uptake of tracer into the brain. Two computed tomography scans were obtained before baseline and drug conditions to correct for attenuation of signal in its passage through bone and cerebral tissue, and to align the PET camera to each volunteer’s brain. The images were reconstructed using filtered back projection and standard clinical protocols, and stored as “counts” images (counts of coincidence events expressed as nCi/cc). The structural (T1) magnetic resonance scan for each participant was used in co-registration and spatial normalization in statistical analysis of brain images.
Study drugs
Placebo solutions had the same appearance as thiopental (multi-vitamin solution in saline) or propofol (intralipid). Both investigators and participants were blinded as to the nature of the study drug.
Computer controlled drug infusion
Study drug was given by intravenous infusion using STANPUMP software controlling a Harvard22 infusion pump (Harvard Apparatus, Holliston, MA). This software produces a constant plasma and/or effect site concentration based on population pharmacokinetics adjusted for weight, age and sex depending on the particular kinetic data set used. Propofol was infused using the Schnider kinetic data-set. Target concentrations were chosen based on previous experience so that propofol would have some degree of memory impairment, with thiopental being given at an equivalent sedative dose. The target concentrations used were propofol 0.9 mcg/ml or thiopental 3 mcg/ml. 2,19,35
Auditory Stimuli
Auditory stimuli were presented using Stim software (Neuroscan, Charlotte, NC). A total of 60 words were presented over a 3 minute time period. Word presentation started 30 seconds before PET imaging began and continued for 60 seconds after scanning was completed. Behavioral results reflect the performance on the total set of words presented. Participants wore foam earphones inserted into the auditory canal (EarLink Auditory Systems, Indianapolis, IN). Words were delivered every 3 seconds at 80 dB SPL. After insertion of earphones and positioning in the scanner participants were tested for clear and bilaterally equal perception of words before imaging was started.
Word Stimuli
Words presented in a given deep encoding task were presented two more times during PET scanning before final EODrcg. Shallowly encoded words were only presented once (during the encoding task) before final EODrcg (see table 1). Separate word lists were prepared for baseline and drug conditions and presented in counterbalanced order across participants. Two-syllable words from the Toronto Word Pool 38 (word frequency <100; mean duration 766 msec) were used. Care was taken to avoid using similar-sounding words as targets and distractors. Words were digitized for computer presentation as female or male voices. Words for the deep encoding task (living/non-living) were presented in a third, neutral voice.‡ At EOD recognition, the stimuli were presented in the same voice used at encoding.
Divided Attention Stimuli
Participants randomized to the placebo/DA condition heard a series of tones randomly interspersed with word stimuli in the drug condition only. Tones and words were presented by two separate computers so that intervals between words and tones were truly random. Tones were of either 1000 or 1100 Hz frequency, 800 msec duration, presented randomly with the high-pitched tones occurring at an overall frequency of 30%. Upon hearing the high-pitched tone, participants pushed a button with the left hand, while still using the right hand for responses to encoding and recognition tasks. All participants in all study groups held both response devices.
Statistical Analysis
In planning of the current study, power analysis of rCBF response determined that 9 participants in each study group would be required to achieve 90% power at alpha =0.01 based on an effect size estimated at 1.5. Unanticipated technical issues with O-15 production resulted in smaller accruals than this desired goal. Thus the power to accept or reject the hypothesis as stated is limited, and results are to be interpreted in light of this consideration.
Behavioral data
Performance accuracy and reaction times on the encoding tasks during PET imaging were obtained as measures of sedation in the drug as compared with the baseline conditions. EODrcg was the primary response variable in testing the memory effects of study manipulations. However, interim memory performance was also assessed using recognition tasks of deeply encoded words with the participant in the PET scanner at approximately 18 and 42 minutes after deep encoding.
Final recognition tasks were administered at the end of the study day. All recognition scores were corrected for false alarms before statistical analysis. False alarms are defined as a new word (distractor) that the participant identified as old (having been presented during PET imaging). Due to the small number of participants, nonparametric Kruskal-Wallis tests for independent samples for the main effect of group (propofol, placebo/DA and thiopental), and Friedman tests for related samples were used to test the effect of stimulus type (shallow or deep encoding words) factors. Within-participant changes (by group and condition) were addressed using non-parametric Wilcoxon signed-rank tests. Data were combined across groups in the baseline condition in certain comparisons, as all participants underwent the same treatment in this condition. A threshold significance of p<0.05 was chosen, although p values near this cut-off are reported as trends due to small sample sizes. Statistical analyses of behavioral data were conducted using SPSS v. 12.0 (Chicago, IL).
Statistical analysis of PET images
Images were reconstructed into. mnc format for analysis. Statistical analysis was performed using Statistical Parametric Mapping 99 § implemented in Pro Matlab v. 7.0, release 14 (Mathworks, New York, NY). PET images were co-registered to the participant’s structural T1 weighted magnetic resonance image.
Images were realigned to the first baseline or drug scan and normalized into Montreal Neurological Institute brain image space with resultant voxel size of 3 × 3 × 3 mm. A 10 mm Gaussian smoothing kernel was used to accommodate interpersonal variations in gyral anatomy and facilitate intersubject averaging with a resultant smoothness of 12.4 by 13.2 by 15.8 (x,y,z) mm. Mean global CBF was normalized to 50 ml • 100−1 g brain tissue • min−1. Statistical analysis employed a proportional scaling model, which allows a differing relationship between regional CBF effect depending on global CBF.
The main statistical parametric mapping contrast was constructed to identify regions of the brain more active in the deep than shallow encoding tasks using images from 17 participants in the baseline condition using a voxel-wise p-value threshold of p<0.001 (see fig. 1). Normally, a region of interest (ROI) is defined on the basis of a priori considerations. In the current study, the paradigm used is almost exactly the same as used by Kapur et al. 24 Rather than using defined anatomical structures, the ROI was critically defined by the contrast of deep versus shallow encoding, where all aspects of the task (e.g. auditory stimulation, button press, etc.) were identical except for cognitive processing. The ROI thus identified in the current study corresponded closely to the results of Kapur and colleagues. Supporting the use of this ROI as an a priori region for statistical analysis is replication of this region in other studies of encoding memory using functional magnetic resonance imaging methods. 13,23,31
Fig 1.
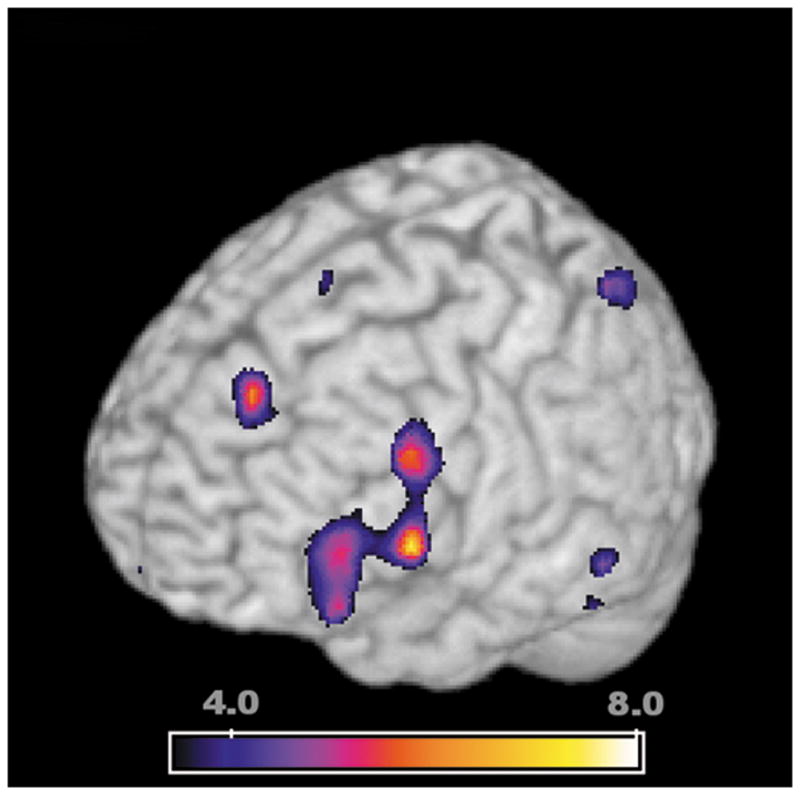
Regions of the brain that were more active in the deep encoding task (semantic, meaning based evaluation of words, “did the word represent a living object?”) than shallow encoding (stimulus based evaluation of words, “was the word spoken in a male or female voice?”). Statistical significance in this map represents greater rCBF as quantified using SPM99 at a voxel-wise p-value threshold of p=0.001 (T=3.55). The regions displayed were identified from the baseline condition using all 17 subjects in the current study. The ROI in the left inferior prefrontal cortex (LIPFC, peak activation at MNI x,y,z co-ordinates of -48,12,3 mm, T=7.44) has been associated with verbal encoding tasks in multiple previous studies. This region was considered an a priori region of interest, as the location of activation is very similarly to that identified by other investigators. A smaller region of activation, not considered as an a priori region of interest, was evident near the midline in the anterior cingulate gyrus (peak activation at −9,39,42, T=6.03). The colored bar represents the T-value significance in this comparison of deep greater than shallow encoding.
This ROI thus identified was used in Statistical Parametric Mapping 99 analyses of images in each treatment group in baseline and drug conditions. A small volume correction was applied to correct for multiple comparisons in the ROI, and measure of significances, i.e. T-values, of activation of deep vs shallow encoding contrasts were used as an estimate of effect size. The deep versus shallow encoding contrast in the baseline condition revealed another region of activation present near the midline, but this was not considered an a priori ROI.
Neuroanatomical labeling of the ROIs was performed using automated anatomical labeling software, which interfaces with Statistical Parametric Mapping 99.**
RESULTS
Participants
Of the 25 recruited, 17 participants (12 M) fully completed the study, with 6 receiving propofol, 5 placebo/DA, and 6 thiopental (see table 2). As noted in the methods, the relatively small numbers in each group did not achieve the planned accrual. Of the eight participants excluded, four fell asleep (responded to less than 50 of 60 word stimuli) during one of the encoding tasks (3 thiopental, 1 propofol), one did not understand instructions, in one the scanner malfunctioned, and 3 were assigned to a placebo/no divided attention group (not analyzed due to insufficient numbers). 13 of the participants were college graduates or had an advanced degree; the remainder had at least some post-secondary education. Mean scores on the Edinburgh Handedness Inventory were strongly right-handed, 88.2 (14.9). Percentile scores on the vocabulary subtest of the Wechsler Adult Intelligence Scale--Revised ranged from 50 to 99, with a mean (SD) of 83.9 (15.9). Eleven participants (64.7%) were Caucasian.
Table 2.
Subject variables using nonparametric descriptors: median (interquartile range).
| GROUP | Gender (M, F) | Age (years) | BMI | WAIS-R Vocabulary | Handedness | Hearing | Sleep (hours) | MRI Day (Same, Different) |
|---|---|---|---|---|---|---|---|---|
| PROP (n=6) | 5, 1 | 30.0 (10.8) | 25.0 (2.1) | 83.0 (49.0) | 92.3 (34.0) | 16.5 (2.8) | 7.0 (1.0) | 3, 3 |
| PLAC/DA (n=5) | 4, 1 | 27.0 (15.0) | 26.2 (7.4) | 84.0 (16.5) | 86.6 (28.0) | 18.0 (3.0) | 6.5 (1.5) | 4, 1 |
| THP (n=6) | 3, 3 | 34.0 (7.8) | 24.1 (3.2) | 95.0 (16.5) | 100.0 (5.0) | 18.5 (3.8) | 7.0 (2.4) | 1, 5 |
| ALL (N=17) | 12, 5 | 31.0 (10.5) | 24.5 (3.5) | 84.0 (23.5) | 100.0 (24.0) | 18.0 (3.0) | 7.0 (1.3) | 8, 9 |
Differences between groups were tested by nonparametric Kruskal Wallis H test or chi squared, as appropriate. The groups did not differ significantly on any variable.
Behavioral Results
The goal of the experimental task, i.e., the levels of processing paradigm, was to ensure encoding of verbal information into long term memory, both in the baseline and drug conditions. Memories had to be of sufficient strength so that recognition would be above chance at the end of the study day. In order to achieve these results, participants had to perform at high levels in the confines of the PET scanner, which might have been difficult especially in the drug condition. Measures of encoding performance demonstrated that participants performed accurately both in baseline and drug conditions. As anticipated, the semantic categorization task proved to be more difficult than speaker voice categorization as measured by accuracy rates and reaction times. This relationship was present in both baseline and drug conditions, and resulted in robust memories for deeply encoded words.
Encoding Accuracy and Reaction Times
Performance during encoding tasks in both baseline and drug conditions was more than 88% accurate, indicating that attention was given to the task in both study conditions (see table 3). There was no significant effect of study group (propofol, placebo/DA or thiopental) on accuracy in the baseline or drug conditions for either stimulus type (Kruskal-Wallis, no significance). Thus all groups performed equally well in both conditions. In the baseline condition (n=17) there was a strong effect present for stimulus type (deep versus shallow words) on accuracy of categorization, with shallow categorizations (96 ± 7.6% correct) being more accurate than deep categorizations (90.5 ± 5.1%) (Friedman, p=0.002). In the group by condition comparisons, significant effects were present in propofol baseline and possibly drug conditions (Wilcoxon signed-rank p=0.03 and p=0.06) thiopental baseline condition (p=0.05) and possibly placebo/DA drug condition (p=0.08), as indicated in table 3.
Table 3.
Percent correct of encoding performance while in the PET scanner.
| Group (N) | Baseline Shallow Encoding | Baseline Deep Encoding | Drug Shallow Encoding | Drug Deep Encoding |
|---|---|---|---|---|
| Propofol (6) | 100 (5.4) | 90.0 (9.1)** | 97.5 (7.5) | 89.2 (11.7)+† |
| Placebo/DA (5) | 95.0 (18.3) | 91.7 (6.7) | 96.7 (9.2) | 88.3 (8.4)+ |
| Thiopental (6) | 98.3 (1.7) | 89.2 (11.2)* | 95.9 (17.0) | 91.7 (8.8) |
Data are shown as median (interquartile range).
Shallow encoding accuracy < deep encoding accuracy by Wilcoxon signed-rank
p<0.05
p<0.10
Also indicative that semantic categorization was a more difficult task were the longer reaction times required to categorize these stimuli as compared to speaker voice determinations. As with encoding accuracy, there was no effect of group (propofol, thiopental, plac/DA) on reaction times in either drug or baseline conditions for either deep or shallow stimulus types (Kruskal-Wallis, no significance). Thus all groups reacted equally fast to categorize stimuli in both conditions. In the baseline condition there is a strong effect of stimulus type on reaction time, with deep categorizations taking longer (1410 ± 216 msec) than shallow categorizations (1097 ± 262 msec)(Friedman, p=0.008). In group by condition testing significantly longer reaction times to deep encoding stimuli were present for placebo/DA baseline condition (Wilcoxon signed-rank, p=0.04) and thiopental baseline (p=0.05, see table 4).
Table 4.
Reaction times (milliseconds) during performance of the encoding task while in PET scanner during imaging.
| Group (N) | Baseline Shallow Encoding | Baseline Deep Encoding | Drug Shallow Encoding | Drug Deep Encoding |
|---|---|---|---|---|
| Propofol (6) | 895 (507) | 1258 (302) | 929 (637) | 1346 (101) |
| Placebo/DA (5) | 1178 (624) | 1684 (438)* | 1196 (404) | 1482 (466) |
| Thiopental (6) | 1046 (221) | 1367 (182)* | 1207 (383) | 1340 (188) |
Data represent correct responses only, and are shown as median (interquartile range).
Indicates reaction time to categorize deep encoding stimuli is greater than shallow encoding (Wilcoxon signed-rank test, p<0.05)
Paired contrasts of condition effect (baseline, drug) for both performance parameters in each group revealed no significant effects, except possibly for encoding accuracy in the placebo/DA group in the deep encoding task (Wilcoxon signed-rank, p=0.06, all others, no signficance). Thus, in a given group, participants had similar behavioral responses to the categorization tasks regardless of whether drug was present of not.
Memory
As revealed in the baseline condition, semantic categorization in the level of processing paradigm was a more difficult task. This resulted in better memory for these stimuli at the end of the study day, as is typical for this paradigm. All recognition scores were corrected for false alarms (new words that were categorized as old) before statistical testing (the false alarm rates are reported in table 5). In all groups, significantly greater memory at the end of the study day for deeply encoded words was present when compared to shallow ones, in both baseline and drug conditions (Wilcoxon signed-rank, p<0.05 for all EODrcg cells in table 5). Essentially, when corrected for false alarms, recognition scores for shallowly encoded words at EOD recognition were zero. Thus EOD recognition memory for deeply encoded words was above chance levels for all groups in both baseline and drug conditions.
Table 5.
Recognitions of deeply encoded words, in PET scanner (Rcg1 and Rcg2) and at end of day (EODrcg).
| Rcg 1 Baseline (scans 3 + 4) | DRUG INFUSION BEGINS | Rcg 2 Baseline 2 (scans 5 + 6) | Rcg 1 Drug (scans 9 + 10) | Rcg 2 Drug (scans 11 + 12) | OUT OF SCANNER | EOD Rcg Baseline Shallow words | EOD Rcg Baseline Deep words | EOD Rcg Drug Shallow words | EOD Rcg Drug Deep words | ||
| PROP
(n=6) |
Score
(IQR) FA (IQR FA) |
75.8
(8.8) 16.7 (11.3) |
90.8
(17.9) 10.8 (7.5) |
60.0
(50.0) 16.7 (19.2) |
59.2
(56.7) 15.0 (10.0) |
33.4
(33.4) 20.0 (25.0) |
83.3*
(28.3) 13.3 (26.7) |
28.4
(34.2) 20.0 (19.2) |
60.0**
(30.8) 20.0 (19.2) |
||
| PLAC/DA
(n=5) |
Score
(IQR) FA (IQR FA) |
78.4
(10.0) 23.3 (20.8) |
91.7
(12.5) 10.0 (23.4) |
75.0
(22.5) 33.3 (23.3) |
95.0
(6.7) 20.0 (11.7) |
26.7
(36.7) 26.7 (40.0) |
93.3*
(18.3) 20.0 (60.0) |
30.0
(41.7) 23.3 (21.7) |
86.7**
(25.0) 16.7 (20.0) |
||
| THP
(n=6) |
Score
(IQR) FA (IQR FA) |
74.2
(12.9) 20.0 (9.6) |
70.9
(30.0) 18.3 (10.9) |
55.8
(25.4) 18.3 (11.3) |
70.0
(10.4) 15.0 (15.4) |
21.7
(35.0) 20.0 (19.2) |
73.4*
(36.7) 18.3 (23.4) |
26.7
(30.9) 21.7 (26.7) |
70.0**
(25.0) 13.3 (12.5) |
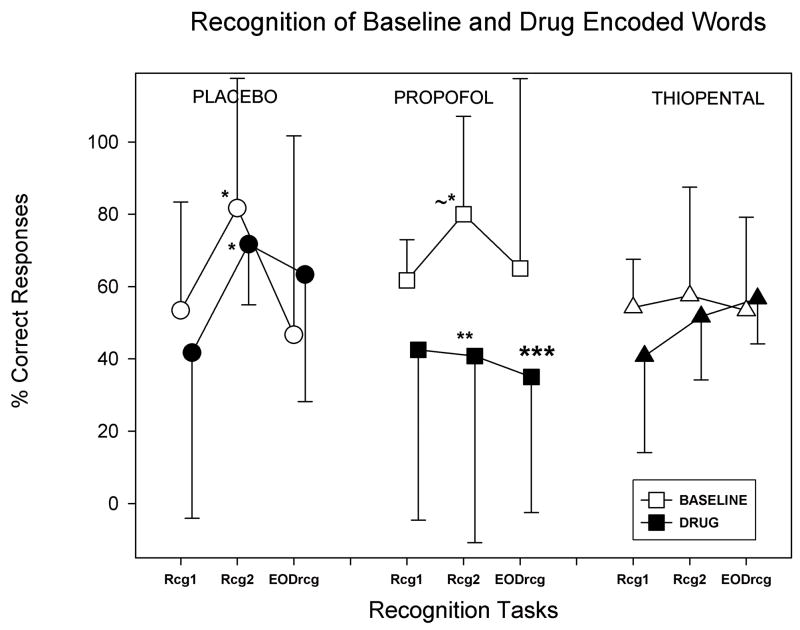
These data are displayed in Figure 2. Recognition of shallowly encoded words was tested at EODrcg only. Scores represent median and interquartile range (IQR), with false alarms in each cell indicated. All scores were corrected for false alarm rate before statistical analysis. EODrcg for deeply encoded words was significantly better than shallowly encoded words in all groups in both conditions (Wilcoxon signed-rank test, p<.05). Values for Rcg1 and Rcg2 are the average of the two old/new tasks used for each PET scan in Table 1.
indicates a significant difference between EOD rcg baseline deep words and EOD rcg baseline shallow words.
indicates a significant difference between EOD rcg drug shallow words and EOD rcg drug deep words
As would be expected by repeated presentations of stimuli in the recognition tasks conducted in the PET scanner soon after encoding, some improvement in memory occurred for deeply encoded words, as represented by differences in recognition2 and recognition1 correct responses in figure 2. A significant learning effect was present on the second recognition task in the placebo/DA group in both baseline and drug conditions (Wilcoxon signed-rank, recognition 1 vs recognition 2, baseline p=0.043, drug p=0.043) and likely also in the propofol, but not thiopental groups in the baseline condition (Wilcoxon signed-rank, recognition 1 vs. recognition 2, propofol p=0.058).
Fig 2.
Recognition memory for deeply encoded words in baseline and drug conditions, corrected for false alarms, over the time of the study. As there are few participants in each group, median values with interquartile ranges were plotted. The key behavioral result of the current study is that final, end of day recognition memory (EODrcg) for words encoded in the presence of propofol is significantly less than for the placebo/DA and thiopental groups (p=0.031, indicated by ***). As there was no change in accuracy of encoding performance or reaction times with propofol, this result is indicative of drug induced amnesia for propofol. Three recognition tasks of words presented in the corresponding deep encoding task occurred for each participant. The first two recognitions (Rcg1 and Rcg2) were performed in the PET scanner. The first recognition (Rcg1) occurred ~18 minutes after encoding, and the second recognition (Rcg2) occurred ~55 min after encoding in the baseline and ~42 min in the drug conditions. A significant learning effect was probably present between rcg1 and rcg2 for the propofol group in the baseline condition (p=0.058, indicated by ~*), and the placebo/DA group in both conditions (p=0.043, indicated by the *). The drug induced amnesic effect of propofol was evident at Rcg2, where memory for deeply encoded words in the presence of propofol was less than in the baseline condition (p=0.028, indicated by **).
The key behavioral result in the current study was a significant effect of group (propofol, placebo/DA, thiopental) on recognition of deeply encoded words at the end of the study day (Kruskal-Wallis, p=0.031). Using planned comparisons, deeply encoded words in the drug condition for propofol were more poorly recognized than baseline encoded words at the end of the day (Wilcoxon signed-rank test, p=0.046). This differential effect of propofol on memory for deeply encoded words was evident in the second recognition testing ~42 minutes after deep encoding in the drug condition (Wilcoxon signed-rank, baseline vs. drug encoded words in recognition 2, p=0.028, as shown in fig. 2, baseline vs. drug in Rcg 2).
To summarize, propofol did not affect reaction time or accuracy during encoding, but resulted in memory impairment at the end of the study day. Divided attention and thiopental manipulations likewise did not affect encoding performance, but as opposed to propofol did not impair memory for deeply encoded words at the end of the study day.
rCBF correlates of Deep encoding
The levels of processing manipulation, being a more difficult task requiring semantic categorization, resulted in memories that were recognized at the end of the study day. PET imaging of brain activity at the time of encoding of these memories revealed increased rCBF in a specific region of the left prefrontal cortex when deep encoding was contrasted with shallow encoding in the baseline condition. Because all groups received the same treatment in the baseline condition, image data from all 17 participants were used to define the a priori ROI used in subsequent analyses.
The largest region of brain activation during deep encoding was indeed in the left inferior prefrontal cortex as previously reported by other investigators. This ROI (roiPFC) comprised a volume of 239 voxels (6.55 cu.cm, cluster level p<0.001 for occurrence of a cluster of voxels this large), with a maximum T value of 7.44 at x,y,z co-ordinates in Montreal Neurologic Institute brain space of −48,12,3 mm (see fig. 1). This region encompassed the middle and inferior frontal gyri, the precentral gyrus and insula and included Brodmann’s areas 44,45,46,47 and 9, 11 and 13.
To address the hypothesis that propofol’s amnesic effect is mediated by an effect that weakens encoding, the response of the roiPFC to deep vs. shallow encoding was tested separately in each group and condition, i.e. a 3 by 2 comparison as shown in figure 3 and table 6. Due to the small numbers in each group, formal contrasts between groups could not be conducted. However, the effect sizes of deep vs. shallow contrasts in the roiPFC (estimated by T values in the statistical parametric maps of these contrasts) are tabulated in table 6. In the baseline condition, significant rCBF increases in the roiPFC was present in each group. Maximum T values of significance for propofol (n=6), placebo/DA (n=5), and thiopental (n=6) groups were 4.88 (p=0.018), 4.41 (p=0.035) and 4.52 (p=0.030) respectively, using small volume correction, which corrects p-values for multiple comparisons in the ROI (see fig. 3). In contrast, in the drug condition, only propofol demonstrated a borderline increased rCBF with deep versus shallow encoding in the roiPFC, with a maximal T value of 4.00 (p=0.063). Corresponding T-values for placebo/DA and thiopental were 2.55 and 2.12, both non-significant.
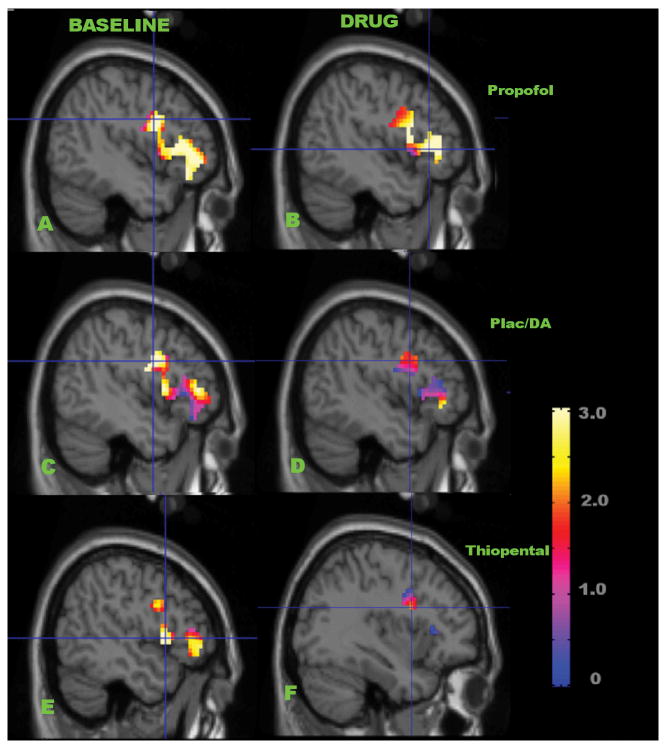
Fig 3.
The region of interest depicted in Fig 1 is shown here for each group in baseline (a,c, and e) and drug conditions (b, d and f). The effect sizes for deep versus shallow encoding activation of rCBF in this region are estimated by T values of significance in these statistical maps, and correspond to the values reported in Table 6 (from top to bottom, propofol (a,b), placebo/DA (c,d), and thiopental (e,f), in baseline (a,c,e) and drug conditions (b,d,f)). The significance of increased rCBF is corrected for multiple comparisons using small volume correction in the region of interest (voxel-wise T-values, colored bar). Note the similarity in activations in the baseline condition for all groups (a,c, and e), and in the drug condition for the propofol group (b). Appreciably smaller activations in the drug condition for placebo/DA and thiopental groups were present (d and f). The key result of the current study was borderline significant activation (p=0.063) during deep encoding with propofol present, as opposed to the placebo/DA and thiopental control groups. The crosshair (thin blue lines) indicates the location of highest significance in each study group in each condition (see Table 6 for these co-ordinates).
Table 6.
| Baseline | Drug | |
|---|---|---|
| Propofol | T= 4.88, p=0.018
x= −44 y= 4 z= 29 |
T= 4.00, p=0.063
x= −45 y= 27 z= 3 |
| Placebo/Divided Attention | T= 4.41, p=0.035
x= −45 y= 0 z= 27 |
T= 2.55, p=0.398
x= −45 y= 7 z= 28 |
| Thiopental | T= 4.52, p=0.030
x= −48 y= 12 z= 3 |
T= 2.12, p=0.579
x= −36 y= 6 z= 24 |
Effect size of deep versus shallow encoding contrasts for increased rCBF in the roiPFC in the LIPFC shown in Figs 1 and 3, as estimated by T-values. The maximum voxel-significance in the roiPFC, corrected for multiple comparisons in this region using small volume correction in SPM99 is tabulated, along with the location in MNI space of that voxel. The most-significant voxel location is indicated in Figure 3 by the crosshairs.
A separate ROI was evident at baseline near the midline and, as described in the methods, cannot be considered an a priori ROI. This ROI (roiACG) consisted of 79 voxels (2.13 cu.cm., cluster level p<0.03) with maximal T value of 6.03 at Montreal Neurological Institute co-ordinates −9,39,42 mm. This region encompassed anterior cingulate gyrus, and superior and medial frontal gyri (BAs 6,8,9 and 32). For each study group at baseline, T values of maximal activity in this ROI were T=3.93 (p<0.02) for propofol, T=2.61 (p=0.12) for placebo/DA, and T=2.86 (p=0.09) for thiopental, using small volume correction for this ROI. In the drug condition only propofol demonstrated increased rCBF in roiACG, with a T value of 3.17 (p=0.056). Essentially no activity was present in the drug condition for placebo or thiopental groups.
DISCUSSION
Using regional cerebral blood flow (rCBF) changes in response to a levels-of-processing (LoP) manipulation of word evaluations, the present study examined the influence of propofol on brain processes underlying the encoding of verbal information into long term episodic memory. At amnesic doses of propofol, encoding activity in the left pre-frontal cortex of the brain was still largely present at a time when behavioral performance on the encoding task was unaffected. To be specific, the amnesic dose of propofol in the current study resulted in memory impairment at the end of the study day but did not impair behavioral measures of encoding performance. Thus, memory impairment was not as a result of sedation, but rather was representative of drug induced amnesia by propofol. Both thiopental and divided attention manipulations influenced activation in the prefrontal cortex, thus demonstrating the sensitivity of the experimental methods to detect changes in this brain region despite small numbers of participants. Thus, drug induced amnesia from propofol did not solely result from impairment of encoding.
The LoP manipulation was successful in creating memories for deeply encoded words that lasted over the length of the study, and was a more important influence than the learning effect from repeated presentations of words on the deep encoding task. This was demonstrated by the fact that final recognition memory for deeply encoded words was better than shallowly encoded words in the thiopental group even though no learning effect was present in that group. These robust memories were likely a result of greater cognitive processing requirements needed for deep encoding, as revealed by longer reaction times and more error prone categorizations. Importantly, the LoP manipulation that resulted in these long lasting memories activated a well-defined region in the left prefrontal cortex during the encoding process. This region maps closely to those previously identified as integral to verbal encoding by other investigators using various neuroimaging technqiues. 5,9,13,23,24,31,39–42 These methods can provide additional insight underlying mechanisms of memory dysfunction, even when task performance itself is unaffected. 10,11
The current study revealed that propofol is unlikely to have its major effect on long term memory by impairing encoding processes. In isolation, the small change in effect size of activation in the LIPFC in the presence of propofol, as seen in the top panels in figure3 would not be revealing. However, when this result is compared to the substantially decreased effect size by divided attention and thiopental manipulations in the drug condition (right sided panels in fig. 3 and table 6), the different nature of the effect of propofol on LIPFC activity is evident. The divided attention manipulation replicates previous findings that show a decrease in LIPFC activity with changes in attention. In those studies, the change in activity in this brain region was related to changes in long term memory, which were felt to be mediated via attentional mechanisms impacting so-called executive functions. 30,32–34 Sub-regions of the LIPFC have been closely associated with working memory, which in turn is closely related to encoding processes. 12,13,15 Previously we have demonstrated that thiopental can interfere with encoding via effects on working memory. Thus, the current study raises the possibility that the LIPFC may be sensitive changes in attention or to sedation.
As we showed previously, and was replicated in the current study, impairment of memory from propofol occurred some time after encoding of information into long-term memory. 19 In comparison with the learning effects present in the placebo/DA group and the baseline response in the propofol group, seen as greater success at recognition 2 than recognition 1 in figure 2, the effect of propofol on memory seemed to start at some point between 18 and 42 min after encoding. This is in line with the imaging findings in the current study that showed propofol did not have much effect on encoding activity in the LIPFC.
We have previously described decreases in rCBF in the prefrontal cortex with amnesic doses of propofol. 43 At first these findings seem incongruous with the presence of encoding activity in the LIPFC as seen in the current study. Despite decreases in CBF with anesthetic agents other investigators have noted that brain activation to stimulation is still present. 44,45 Thus increased rCBF in the LIPFC during deep encoding can be consistent with the decreases in rCBF we previously reported with propofol. Retention of episodic memories is dependent upon a network of brain regions, most notably prefrontal, hippocampal/medial temporal, and parietal regions. 6,7,46–48 The word memory task used in our previous study did not isolate encoding processes, as in the current study, and resulted in increased rCBF in both prefrontal and parietal regions. It is possible that the effect of propofol on memory may have been mediated via actions in the parietal rather than the pre-frontal region. This possibility is consistent with changes in event related potentials measures of recognition memory in the parietal region soon after encoding in the presence of propofol, preliminary results of which we have recently presented. 20 Thus, the effect of propofol on memory processes and neuroanatomical structures underlying its amnesic actions may be more evident in recognition tasks, particularly those involving parietal regions.
In the current study there was dissociation between final recognition memory at the end of day and LIPFC activity at encoding. In contrast with propofol, there was no change in final recognition memory in the divided attention and thiopental groups, despite the lack of activation in the LIPFC in the drug condition. A number of possibilities can be put forward to explain this observation. In the presence of weaker encoding, as indicated by decreased response in the LIPFC in the thiopental and plac/DA groups, recognition of words at the end of the study day may have engaged different memory processes than normally used. For example familiarity (knowing one has experienced something) may have resulted in recognition instead of recollection (a sense of distinct recall), as occurs in the elderly. 10 The use of alternative recognition processes was not available for the participants who received propofol as any memories were degraded by that time, i.e. there was not even a weak trace available for recognition of most memories. Results from the thiopental group raise the possibility that LIPFC activity may be influenced by sedation. As non-sedative doses of propofol were used in the current study little influence on LIPFC activation might have been expected if this had been the case. On the other hand, participants receiving propofol may have compensated for the presence of drug with increased attention during encoding, as has been demonstrated in memory function in the elderly. 49 Such cognitive effort may have counteracted an underlying effect to diminish LIPFC activity.
Activity in the hippocampus rather than the LIPFC may relate more directly to subsequent memory. 50 Despite the fact that we didn’t find changes in rCBF in the medial temporal lobe in this and in our previous study, animal studies suggest that propofol requires the amygdala to exert its amnesic effect. 43,51 Thus, methods which identify the hippocampus/medial temporal lobe, such as the subsequent memory paradigms used in event-related functional magnetic resonance imaging studies, may help resolve this issue. 13,31,39,41
There are certain limitations, which should be kept in mind when interpreting the findings from the current study. The largest is the unanticipated small accrual. This was problematic in that sufficient power was not present to allow recognition-related brain activity to be imaged, which may have provided valuable insight into propofol’s actions on memory after encoding. Fortunately, this wasn’t the case for encoding activity, as the LoP paradigm was sufficiently robust to allow identification of brain activity in the baseline condition in all groups. The use of multiple recognitions of deeply encoded words, as compared to a single recognition for shallowly encoded words may have exaggerated differences in memory between these items. Thus, the final memory effect of propofol is affected by multiple influences beyond those present during encoding, and caution should be exercised in linking memory on final recognition testing and brain activity at encoding. It should be noted that there was some decrease in effect size in the propofol group in the drug condition, though not as large as that present in the control groups. Thus, the possibility still exists that propofol has some influence, albeit not a major one, on the prefrontal cortex.
In conclusion, these initial observations of brain activity during encoding of auditory words reveal no appreciable effect of amnesic, but not sedative concentrations of propofol on the left prefrontal cortex. The current study supports findings from our previous studies where encoding of information into long-term memory in the presence of propofol appears to be normal. As opposed to that which occurs in the elderly, the nature of encoding processes underlying normal encoding performance in the presence of propofol appears largely unaffected. However, the LIPFC region of the pre-frontal cortex was affected by a divided attention manipulation or the administration of thiopental. Thus, this region of the prefrontal cortex may be sensitive to changes in attention or sedation during encoding. In light of the fact that amnesic doses of propofol had little effect on the response of the prefrontal cortex to encoding of verbal information into long term memory, one can conclude that the production of amnesia at these low doses of propofol does not solely reside in interference with encoding processes, nor involve an isolated action on the prefrontal cortex.
Acknowledgments
Funding support from National Institutes of Health, R01 GM58782, Bethesda, Maryland and Departmental Research Funds
We wish to thank the staff of the Citigroup Biomedical Imaging Center, New York, New York for assistance in collection of imaging data. We thank Steven Shafer. M.D., Professor of Anesthesiology, Columbia University, New York, NY for the use of STANPUMP. We thank Brad Beattie, M.Sc., Technical Director Animal Imaging Core, Dept of Neurology, Memorial Sloan Kettering Cancer Center, New York, NY and Vladimir Feshchenko, Ph.D., currently Senior IT Consultant, KPMG, Moscow, Russia who helped design experimental set-ups and in collection of the data. We thank Yuelin Li, Ph.D., Assistant Attending Behavioral Scientist, Dept. of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, New York, New York for helpful suggestions regarding statistical analysis of data.
Footnotes
STANPUMP program. Available at http://anesthesia.stanford.edu/pkpd (last accessed 10/05/2007)
Only deeply encoded words, and not shallowly encoded ones, were presented for recognition in the PET scanner, due to the limitations on the number of scans that one participant could undergo.
The computer-generated “Frank” voice from Cepstral Text-to-Speech, Pittsburgh, PA, http://www.cepstral.com/, last accessed April 08, 2008.
Wellcome Institute of Cognitive Neurology, London, United Kingdom http://www.fil.ion.ucl.ac.uk/spm/software/spm99/, (last accessed April 3, 2008)
(http://www.cyceron.fr/freeware/last accessed April 3, 2008).
Summary Statement: Propofol does not change regional cerebral blood flow measures of deep encoding in the left inferior prefrontal cortex, despite production of long term memory deficit.
Presented in part at: Organization for Human Brain Mapping 2005, Jun 14–18, 2005, Toronto, Ontario, Canada and American Society of Anesthesiologists annual meeting Atlanta Oct 22–26, 2005.
References
- 1.Nordstrom O, Sandin R. Recall during intermittent propofol anaesthesia. British Journal of Anaesthesia. 1996;76:699–701. doi: 10.1093/bja/76.5.699. [DOI] [PubMed] [Google Scholar]
- 2.Veselis RA, Reinsel RA, Feshchenko VA, Wronski M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology. 1997;87:749–64. doi: 10.1097/00000542-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Deeprose C, Andrade J, Varma S, Edwards N. Unconscious learning during surgery with propofol anaesthesia. Br J Anaesth. 2004;92:171–7. doi: 10.1093/bja/aeh054. [DOI] [PubMed] [Google Scholar]
- 4.Tulving E. Episodic memory and common sense: how far apart? Philos Trans R Soc Lond B Biol Sci. 2001;356:1505–15. doi: 10.1098/rstb.2001.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microscopy Research and Technique. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Nyberg L, Persson J, Habib R, Tulving E, McIntosh AR, Cabeza R, Houle S. Large scale neurocognitive networks underlying episodic memory. J Cogn Neurosci. 2000;12:163–73. doi: 10.1162/089892900561805. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent Spontaneous Activity Identifies a Hippocampal-Parietal Memory Network. J Neurophysiol. 2006;96:3517–31. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 8.Morcom AM, Good CD, Frackowiak RSJ, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–29. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- 9.Nessler D, Johnson R, Jr, Bersick M, Friedman D. On why the elderly have normal semantic retrieval but deficient episodic encoding: a study of left inferior frontal ERP activity. Neuroimage. 2006;30:299–312. doi: 10.1016/j.neuroimage.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Nessler D, Johnson R, Jr, Bersick M, Friedman D. Age-related ERP differences at retrieval persist despite age-invariant performance and left-frontal negativity during encoding. Neurosci Lett. 2008;432:151–6. doi: 10.1016/j.neulet.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30:529–38. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 12.D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- 13.Staresina BP, Davachi L. Differential Encoding Mechanisms for Subsequent Associative Recognition and Free Recall. J Neurosci. 2006;26:9162–72. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganath C, Cohen MX, Brozinsky CJ. Working Memory Maintenance Contributes to Long-term Memory Formation: Neural and Behavioral Evidence. J Cogn Neurosci. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- 15.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–25. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–5. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 17.Peterson LR, Peterson MJ. Short-term retention of individual verbal items. J Exp Psychol. 1959;58:193–8. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- 18.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 19.Veselis RA, Reinsel RA, Feshchenko VA, Johnson R., Jr Information loss over time defines the memory defect of propofol: A comparative response with thiopental and dexmedetomidine. Anesthesiology. 2004;101:831–41. doi: 10.1097/00000542-200410000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veselis RA, Pryor KO, Reinsel RA, Mehta M, Johnson R., Jr Memory in the presence of Propofol of Midazolam – 30 seconds makes a difference. Br J Anaesth. 2008;100 In Press. [Google Scholar]
- 21.Kahn I, Pascual-Leone A, Theoret H, Fregni F, Clark D, Wagner AD. Transient Disruption of Ventrolateral Prefrontal Cortex During Verbal Encoding Affects Subsequent Memory Performance. J Neurophysiol. 2005;94:688–98. doi: 10.1152/jn.01335.2004. [DOI] [PubMed] [Google Scholar]
- 22.Habib R, Nyberg L. Neural Correlates of Availability and Accessibility in Memory. Cerebral Cortex. 2007 doi: 10.1093/cercor/bhm201. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–17. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 24.Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proceedings of the National Academy of Sciences of the U S A. 1994;91:2008–11. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockhart RS, Craik FIM. Levels of processing: A retrospective commentary on a framework for memory research. Canadian Journal of Psychology. 1990;44:87–112. [Google Scholar]
- 26.Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- 27.Curran HV, Schiwy W, Eves F, Shine P, Lader M. A “levels of processing” study of the effects of benzodiazepines on human memory. Human Psychopharmacology. 1988;3:21–5. [Google Scholar]
- 28.Bishop KI, Curran HV. Psychopharmacological analysis of implicit and explicit memory: a study with lorazepam and the benzodiazepine antagonist flumazenil. Psychopharmacology. 1995;121:267–78. doi: 10.1007/BF02245638. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes MA, Moscovitch M. Divided attention and memory: evidence of substantial interference effects at retrieval and encoding. J Exp Psychol Gen. 2000;129:155–76. doi: 10.1037//0096-3445.129.2.155. [DOI] [PubMed] [Google Scholar]
- 30.Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI. The effects of divided attention on encoding- and retrieval-related brain activity: A PET study of younger and older adults. J Cogn Neurosci. 2000;12:775–92. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- 31.Uncapher MR, Rugg MD. Fractionation of the Component Processes Underlying Successful Episodic Encoding: A combined fMRI and Divided Attention Study. J Cogn Neurosci. 2008;20:240–54. doi: 10.1162/jocn.2008.20026. [DOI] [PubMed] [Google Scholar]
- 32.Iidaka T, Anderson ND, Kapur S, Cabeza R, Craik FI. The effect of divided attention on encoding and retrieval in episodic memory revealed by positron emission tomography. J Cogn Neurosci. 2000;12:267–80. doi: 10.1162/089892900562093. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain. 1995;118:401–16. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. I Encoding Brain. 1998;121(Pt 7):1239–48. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- 35.Pryor KO, Veselis RA, Reinsel RA, Feshchenko VA. Enhanced visual memory effect for negative versus positive emotional content is potentiated at sub-anaesthetic concentrations of thiopental. Br J Anaesth. 2004;93:348–55. doi: 10.1093/bja/aeh211. [DOI] [PubMed] [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Coren S, Hakstian AR. The development and cross-validation of a self-report inventory to assess pure-tone threshold hearing sensitivity. J Speech Hear Res. 1992;35:921–8. doi: 10.1044/jshr.3504.921. [DOI] [PubMed] [Google Scholar]
- 38.Rubin DC, Friendly M. Predicting which words get recalled: measures of free recall, availability, goodness, emotionality, and pronunciability for 925 nouns. Mem Cognit. 1986;14:79–94. doi: 10.3758/bf03209231. [DOI] [PubMed] [Google Scholar]
- 39.Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–80. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 41.Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex. 2001;11:1150–60. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- 42.Friedman D, Trott C. An event-related potential study of encoding in young and older adults. Neuropsychologia. 2000;38:542–57. doi: 10.1016/s0028-3932(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 43.Veselis RA, Reinsel RA, Feshchenko VA, Dnistrian AM. A neuroanatomical construct for the amnesic effects of propofol. Anesthesiology. 2002;97:329–37. doi: 10.1097/00000542-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Ramani R, Qiu M, Constable RT. Sevoflurane 0.25 MAC preferentially affects higher order association areas: a functional magnetic resonance imaging study in volunteers. Anesth Analg. 2007;105:648–55. doi: 10.1213/01.ane.0000277496.12747.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shtoyerman E, Arieli A, Slovin H, Vanzetta I, Grinvald A. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J Neurosci. 2000;20:8111–21. doi: 10.1523/JNEUROSCI.20-21-08111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–78. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 47.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–50. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn059. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 50.Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information [In Process Citation] Proc Natl Acad Sci U S A. 1998;95:14506–10. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alkire MT, Vazdarjanova A, Dickinson-Anson H, White NS, Cahill L. Lesions of the basolateral amygdala complex block propofol-induced amnesia for inhibitory avoidance learning in rats. Anesthesiology. 2001;95:708–15. doi: 10.1097/00000542-200109000-00025. [DOI] [PubMed] [Google Scholar]