Abstract
Exposure to higher levels of both exogenous and endogenous hormone is associated with breast cancer risk. Because of the association between breast cancer and HRT, only the minimal duration of HRT use is recommended for symptom control, and it is not recommended for chronic disease management. Current research issues include the role of progestins, other types of HRT, duration of unopposed estrogen use, and characteristics of cancers that develop on HRT. Circulating sex steroid levels are associated with breast cancer risk, but multiple issues need to be addressed before they are used routinely in clinical practice. Current research issues include measurement of levels for routine clinical practice, integration with standard breast cancer risk models and genetic polymorphism data, and applicability to estrogen-receptor-negative cancers.
Keywords: breast cancer, hormone replacement therapy, estrogen, sex steroids
For over 100 years – since Dr George Beatson described the regression of metastatic breast cancer after an oophorectomy1 – it has been recognized that breast cancer is strongly influenced by hormonal factors. In the 1960s and 1970s, reproductive risk factors such as ages at menarche, first birth, and menopause, and parity were also found to be associated with breast cancer risk and were synthesized by Dr Malcolm Pike into an age–incidence model for breast cancer.2 Later, it became recognized that other breast cancer risk factors such as postmenopausal obesity/weight gain and alcohol consumption also operate through a hormonal mechanism. Finally, the role of exogenous hormones in the form of hormone replacement therapy (HRT) on breast cancer incidence is now being appreciated.3 This chapter will review the influence of both endogenous and exogenous hormones on breast cancer risk.
HORMONE REPLACEMENT THERAPY AND BREAST CANCER
Until the results from the randomized Women’s Health Initiative (WHI) trial in the United States were released in 2002, HRT was widely used by postmenopausal women for a variety of reasons, including menopausal symptoms and prevention of chronic diseases such as cardiovascular disease and osteoporosis. After the publication of the WHI, and shortly thereafter the observational Million Women’s Study from the United Kingdom,4 multiple professional societies worldwide changed their HRT prescribing guidelines and recommended only short-term use, if at all. Consequently, HRT use in the United States5 and Europe6,7 decreased dramatically. However, many women feel frustrated regarding the lack of efficacy of non-hormonal alternatives, such as selective serotonin reuptake inhibitors (SSRIs) and gabapentin for control of menopausal symptoms, so it is important to quantify the magnitude of risk associated with HRT use. When reviewing the literature, it is also crucial to separate the studies evaluating combination estrogen and progesterone therapy and unopposed estrogen, and also to consider the types of progesterone and estrogen utilized since they can vary substantially in terms of their potency and composition.
Main results of the randomized clinical trial: Women’s Health Initiative
The largest randomized placebo-controlled trial evaluating the overall health effects of HRT was the WHI. This was a large, multicenter trial conducted in the United States that randomized 16,608 postmenopausal women with a uterus to either placebo or the combination of 0.625 mg conjugated equine estrogen and 2.5 mg medroxyprogesterone acetate daily (E+P)8 and 10,739 postmenopausal women without a uterus to either 0.625 mg daily of conjugated equine estrogens (E alone) or placebo.9 After a mean follow-up of only 5.2 years, the study was unblinded for the E+P arm alone when event rates for breast cancer and a global index for ‘overall harm’ exceeded predetermined stopping rules.8 With a mean follow-up of 5.6 years, there was a 24% increase in the risk of invasive breast cancer with E+P compared to placebo (95% CI 1.10–1.54, P = 0.003), with the risk becoming apparent in the third year of use among women who had previously used HRT and by the fourth year of use among women who had never used HRT. It was also noted that use of E+P was associated with a higher risk of an abnormal mammogram10 and increased breast density11 even after only 1 year of use. In contrast, there was no increased breast cancer risk in the E alone arm compared to placebo, even after an average of 7.1 years of follow-up. In fact, there was a non-significant decrease in breast cancer risk (RR 0.80; 95% CI 0.62–1.04, P = 0.09). However, similarly to E+P, there was an increased risk of an abnormal mammogram for women using E alone.12 Data on mammographic density for the E-alone arm has not been published to date. On the basis of the WHI data and multiple observational studies,4,13 most professional societies do not recommend HRT for chronic disease prevention, and recommend minimizing exposure when HRT is used for treatment of menopausal symptoms.14,15
Use of HRT in breast cancer survivors
Many breast cancer survivors develop menopausal systems, whether as a consequence of treatment-induced menopause or side-effects of treatment. SSRIs and other non-hormonal interventions may provide some relief, but are still inferior to HRT for treatment of menopausal symptoms. Two small prospective studies – which were closed early due to slow accrual and concerns regarding HRT use in the breast cancer population – did not observe an increased recurrence risk with HRT, but were clearly underpowered.16,17 However, the HABITS trial from Stockholm, beginning in 1997, enrolled 447 breast cancer survivors and assigned them to either hormone therapy (health-care provider’s choice) or none, and was terminated in December 2003 when the event rate in the HRT exceeded predetermine stopping rules. However, follow-up was continued, and at 4 years median follow-up and 56 events, the HRT group was found to have over twice the risk of breast cancer recurrence compared to controls, with absolute differences in event rates at 2 and 4 years of 5.7% (95% CI 3.5–7.9) and 14.2% (95% CI 10.9–17.5%) respectively.18 Results were similar regardless of estrogen receptor (ER) status of the tumor and tamoxifen use, but power was limited for the subgroup analyses. Therefore, among breast cancer survivors, regardless of the ER status of the tumor, even short-term HRT use would not be recommended.
Unanswered questions on exogenous estrogens and breast cancer
Effects of progestins
Before the release of the WHI results, many had assumed that the increased breast cancer risk seen in the observational studies of HRT were due to the effects of estrogens, and many of the earlier studies did not separately analyze the types of HRT regimens. However, with the results of the WHI, focus has shifted to progesterone. It is hypothesized that progesterone may increase cell division and thereby lead to the accumulation of DNA damage. For example, proliferative activity is noted to be highest during the luteal phase of the menstrual cycle, a time when endogenous progesterone levels are high. However, in-vivo and in-vitro studies have not always been consistent in their findings about whether exogenous progesterone increases proliferation in breast tissue.19
Longer duration of unopposed estrogen
Although the WHI did not see an association between unopposed estrogen alone and breast cancer risk with an average follow-up of 7.1 years, the effect of longer-term use of unopposed estrogen and breast cancer risk still needs to be considered. In both the combined analysis of 51 epidemiological studies led by the Oxford group and in the large observational Nurses’ Health Study (NHS) cohort (which was included in the Oxford pooled analysis), no increase in breast cancer risk was seen with less than 5 years of unopposed estrogen. However, with more than 5 years of current estrogen-alone use, the Oxford group reported a pooled RR 1.34 (S.E. 0.09).13 In the Nurses’ Health Study, when we analyzed only women who had undergone a hysterectomy, similarly to the WHI, we did not observe an increased risk of breast cancer with shorter periods of use. However, with much longer durations of use we did observe an increased risk of breast cancer: RR 1.42 (95% CI 1.13–1.77) for 20+ years of use. When limited to ER+/PR+ cancers, we observed an increase in breast cancer after 15 years of current use of unopposed estrogen (RR 1.48; 95% CI 1.05–2.07).20 Therefore, for durations of unopposed estrogen use similar to that in the WHI (i.e. <7 years), there does not appear to be an increased breast cancer risk. However, the impact for longer-term users is less clear.
Characteristics of breast cancer that develop on HRT
In the WHI, the women who took combination estrogen and progesterone (E+P) had a greater chance of being diagnosed with a node-positive or more advanced stage breast cancer than those on placebo.10 This finding has not been consistently seen in the observational studies, and the clinical significance of these findings is not clear. Interestingly, there was no difference seen in the ER and PR (progesterone receptor) status of the tumors between the placebo and the E+P arms, contrary to what was seen in the observational studies and what physiologically would seem most logical. It is well known that medications that block the estrogen receptor, such as tamoxifen, or lower estrogen levels, such as the aromatase inhibitors, only affect the growth of hormone-receptor-positive cancers, but not hormone-receptor-negative ones. Therefore, it would seem reasonable to hypothesize that HRT would preferentially stimulate the growth of hormone-receptor-positive cancers, as was seen in multiple observational studies.21 Part of the discrepancy may be that the WHI study had only a limited number of breast cancer cases with known ER status (182 on E+P and 127 on placebo). In addition, both ER and PR status was selectively more likely to be missing among the placebo group as compared to the E+P group, introducing a potential source of bias. Therefore, although no difference was seen in the distribution of tumors by hormone receptor status in the WHI, given the biological mechanism and the additional power of the observational studies to evaluate differences by receptor status, it is still likely that HRT is more strongly associated with hormone-receptor-positive cancers than negative ones.
Other forms of HRT
In the United Status the most common form of prescription HRT is conjugated equine estrogens given alone or with medroxyprogesterone acetate. However, in Europe, many other formulations of estrogens and progestagens are used, and their effects on breast cancer have not been as well quantified. It should be noted that none of these studies were randomized, and many had only limited numbers of users of the other types of hormones.
Estrogen
The single largest prospective study conducted in Europe was the Million Women’s Study (MWS) in the UK. This study did not observe any variation in risk between conjugated estrogens and ethinylestradiol or for the mode of delivery (oral/transdermal/implanted). In addition, it also reported an increased risk of breast cancer with tibolone, a progestin analog that is considered a selective estrogen enzyme modulator.4 Another prospective study done in Denmark, in which the predominant estrogen used was estradiol rather than conjugated estrogens, also observed an increased risk with estrogen-only and combination E+P regimens and tibolone.22 However, it should be noted that the risk of breast cancer observed with estrogen-only in both of the studies was higher than that seen in the WHI. Both ethinylestradiol and estradiol would be considered ‘medium-potency’ estrogens similar to conjugated estrogens. None of the large studies described above had sufficient power to evaluate doseS of conjugated equine estrogens <0.625 mg daily or lower-potency estrogens.
Progesterone
Interest has also focused on the type of progesterone. The WHI utilized medroxyprogesterone acetate, a synthetic progesterone. In contrast, the prospective French E3N cohort analyzed the association with natural progesterone and did not observe an increased breast cancer risk.23 However, other large studies that evaluated more androgenic progestins, such as norethisterone and norgestrel, still observed an increased breast cancer risk when given in combination with estrogens.4,22 In sum, further research needs to be done to determine whether differences in the cancer risk vary according to the type and properties of the progesterone used.
Testosterone
Only a few studies have evaluated the use of testosterone-based HRT, so power has been limited. In the largest prospective study of oral testosterone given alone or in combination with oral estrogen (n = 32) to date, we observed over a two-fold increase risk of breast cancer within the Nurses’ Health Study compared to never users (multivariate RR 1.77 for current users; 95% CI 1.22–2.56).24 An increased risk was also seen in a Danish study evaluating injectable estrogen and testosterone.25 Finally, as described in the following section, higher endogenous testosterone levels have been associated with increased breast cancer risk in pre- and postmenopausal women. Therefore, although data are only preliminary, testosterone-based HRT should be used with caution in postmenopausal women.
SEX STEROIDS AND BREAST CANCER
Given the associations between breast cancer and HRT and other hormonal risk factors, the role of endogenous sex steroids and breast cancer risk would be an important avenue of investigation. Specifically, many of the reproductive risk factors that increase breast cancer risk (later age at menopause, earlier age at menarche, etc) imply that longer duration of estrogen exposure would increase breast cancer risk. Therefore, it would be reasonable to hypothesize that endogenous hormone levels may represent a final common pathway and function as a biomarker encapsulating a woman’s hormonal exposure over her lifetime. However, the clinical utility of sex steroid assays has been limited by the fact that most clinical laboratories do not have assays sensitive enough to reliably quantify the low circulating levels of estradiol in postmenopausal women. In addition, premenopausal women represent an additional challenge since there is a large amount of intra- and inter-individual variation in circulating hormone levels according to the menstrual cycle. First, I will summarize studies evaluating surrogates for endogenous estrogen exposure, such as body mass index (BMI) and bone density, then focus on studies measuring actual levels.
Surrogates for lifetime estrogen exposure
Body mass index
Multiple studies have linked an increased risk of breast cancer with postmenopausal obesity and weight gain. For example, a pooled analysis of seven prospective cohort studies, mainly from North America, reported a multivariate RR of 1.27 (95% CI 1.03–1.55) for women with a BMI ≥33 kg/m2 compared to those with a BMI<21 kg/m2.26 Similar results were seen in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.27 Within the Nurses’ Health Study (NHS), in addition to current BMI, weight change since age 18 was specifically evaluated, and a 45% increased risk (95% CI 27–66%) was seen among women who gained at least 25 kg since age 18.28
BMI is highly correlated with circulating estrogen levels, and is considered a surrogate for endogenous estrogen exposure. For example, in the Endogenous Hormones and Breast Cancer Collaborative Group (EHBCCG) pooled analysis of eight prospective studies, the mean estradiol level among women with BMI <22.5 kg/m2 was 30.0 pmol/L compared to 54.9 among women weighing ≥30 kg/m2. The increase in postmenopausal breast cancer risk seen with obesity is thought to be almost entirely driven by the increased aromatization of androgens to estrogens in adipose tissue and a decrease in serum sex-hormone-binding globulin levels. In the EHBCCG analysis, the increased risk of breast cancer with increasing BMI could be almost completely explained by adjusting for serum estrogen levels. In contrast, adjustment for androgens had little effect on the relative risk.29 Finally, the lower levels of sex-hormone-binding globulin seen with increasing BMI would lead to higher free (unbound) estradiol levels in heavier women.30
Bone density
Bone density has also been considered as another possible surrogate for endogenous estrogen exposure, although the data are less clear than with BMI. Bone tissue contains estrogen receptors, and exogenous estrogens are well known to increase bone density. Although multiple studies have reported an increased risk of breast cancer with increased bone density, with women in the highest quartile of bone density having roughly 2–3 times the risk of women in the lowest quartile,31,32 several studies did not find a strong association.33
Circulating hormone levels
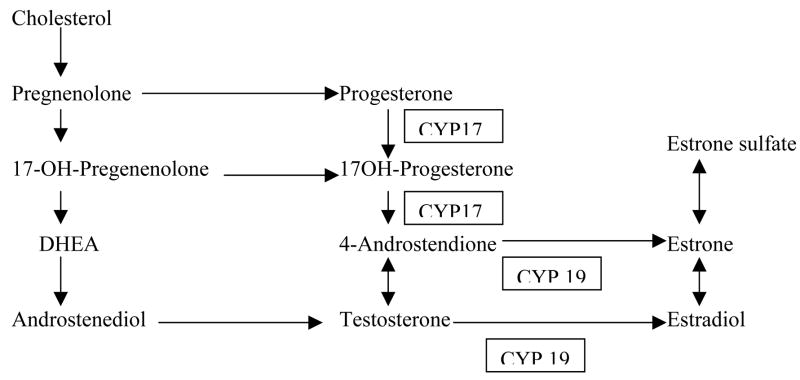
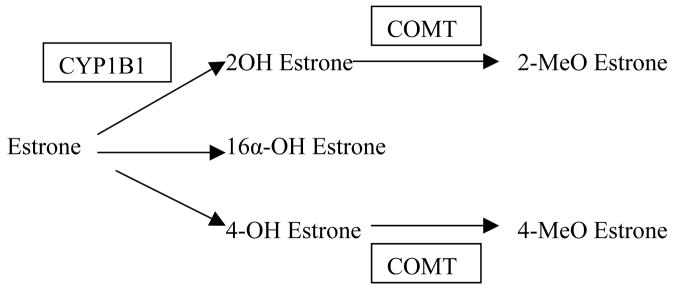
The hope with circulating sex steroid levels would be that they represent the best overall measure of endogenous estrogen exposure and a final common pathway for all the different risk factors that may influence a woman’s breast cancer risk throughout her lifetime, and may be the ‘best’ breast cancer risk predictor. Although the data for postmenopausal women are stronger than for premenopausal women, the use of blood estrogen levels in the clinical setting is still far from routine for a number of reasons, including inter-laboratory variability, especially for the low levels of circulating estrogens in postmenopausal women.34 Many of the earlier studies focused on circulating estrogen levels, but more recent studies have also evaluated androgen levels (Figures 1 and 2).
Figure 1.
Estrogen synthesis. DHEA, dehydroepiandrosterone. Adapted from Kendall et al (2007, Journal of Steroid Biochemistry and Molelcular Biology 103: 99–109) with permission.
Figure 2.
Estrogen catabolism. Adapted from Kendall et al (2007, Journal of Steroid Biochemistry and Molelcular Biology 103: 99–109) with permission.
Postmenopausal women
The body of evidence supporting a link between circulating estrogen levels and breast cancer risk for postmenopausal women are more consistent than for premenopausal women.35–37 For example, in the EHBCCG analysis of nine prospective studies of endogenous hormone levels and breast cancer risk, levels of multiple sex steroids – including total estradiol, free estradiol, estrone, and estrone sulfate – were associated with increased breast cancer risk. In general, those in the highest quintile of circulating hormone levels had twice the risk compared to those in the lowest quintile.36 Similar results were seen within EPIC.38 The Nurses’ Health Study further evaluated the association by hormone receptor status of the tumor, and as expected a stronger association with circulating estrogen levels was found with ER+/PR+ tumors.37
Among postmenopausal women not currently on HRT, a single blood sample seems to be a fairly good reflection of longer-terms levels, based upon reproducibility studies in which several samples were collected over time.39 Furthermore, many of the prospective studies had blood samples drawn years earlier and were still able to predict breast cancer risk.37 In addition to circulating estrogens, many of these studies also observed an association between breast cancer risk and circulating androgens, such as testosterone, androstenedione, and DHEA. The risk associated with circulating androgens appeared to be independent of that with circulating estrogens, since associations were still observed after mutual adjustment.36–38 It should be noted that all of the studies excluded women who were taking HRT. Interestingly, despite the data on exogenous progesterone use and breast cancer risk, endogenous progesterone levels have not been consistently associated with increased breast cancer risk in postmenopausal women.37
Premenopausal women
Data linking circulating estrogen levels and premenopausal breast cancer have been less clear, but these studies have been more difficult to analyze because of large intra-individual variation related to the menstrual cycle and inter-individual variability. Stronger evidence exists for associations between breast cancer risk and premenopausal androgen levels. The largest prospective study to date is EPIC, which reported an association between breast cancer and premenopausal levels of testosterone, androstenedione, and DHEAS (RR comparing highest to lowest quartile range 1.48–1.73), but not for estradiol or estrone.40 However, another large prospective study (the Nurses’ Health Study, NHS) presented slightly different results. It should be noted that the NHS blood samples were timed to the menstrual cycle, rather than simply matching cases and controls to the day of the menstrual cycle as was done in EPIC. Similarly to EPIC, the NHS investigators also observed an association with breast cancer risk and premenopausal testosterone levels. In contrast to EPIC, they also observed an increased breast cancer risk with estradiol levels drawn in the early follicular phase. In premenopausal women, estrogen levels are generally lower in the early follicular phase.41 In summary, for premenopausal women, the associations with circulating androgens appears to be stronger than the data for circulating estrogens, but it is not known how much of the observed differences are due to measurement issues, since androgens can be more reliably measured and have less variation according to the menstrual cycle than estrogens.
Unanswered questions on sex steroids and breast cancer
Laboratory assay methods
From the standpoint of risk prediction, the field of breast cancer could follow the lead of cardiovascular disease in which both epidemiological risk models (such as the Framingham risk score) and biomarkers (such as C-reactive protein and lipid levels) are used to target interventions according to the risk of disease development. However, several issues need to be overcome before circulating sex steroid levels are used routinely in clinical practice. As noted previously, measurement of low estradiol levels require ultra-sensitive assays that even in capable hands at academic research laboratories still do not provide reproducible and comparable absolute levels. For example, in the EHBCG analysis of nine prospective studies, median levels for estradiol ranged from 21.7 to 101 pmol/L among the controls.29 Therefore, study-specific cutoffs were used, but in order to be adopted for widespread clinical use, standard absolute values for the levels will need to be agreed upon and reproducible. Although some inter-individual variability is to be expected, much of the variation in published studies is felt to be due to laboratory variation. Despite the heterogeneity in the laboratory assays, there was little heterogeneity in the association between hormone levels and breast cancer across studies, underscoring the strength of the association with breast cancer risk.
Integration with epidemiological risk prediction models
Currently, the Gail model is the most widely available model for breast cancer risk prediction; this utilizes several standard breast cancer risk factors (age, race, ages at menarche and first live birth, family history of breast cancer, number of benign breast biopsies, and history of atypical hyperplasia) to estimate absolute 5-year risks of developing invasive breast cancer.42 Although circulating sex steroid levels add additional information to risk prediction even after controlling for standard breast cancer risk factors, it is still not clear how to integrate the epidemiological and biomarker data together. In the Nurses’ Health Study, it has been previously shown that even after stratifying by Gail model score, estradiol, estrone, and testosterone levels were still associated with an increased risk and therefore were important as independent risk predictors.43
In addition, data on the use of hormone levels to identify women as candidates for chemoprevention has been mixed. The chemoprevention trials utilizing tamoxifen and raloxifene have all shown that these drugs only target the formation of hormone-receptor-positive cancers, but not hormone-receptor-negative ones, and the Gail model was used as part of the eligibility criteria for the NSABP P-1 (tamoxifen versus placebo among healthy high-risk women) and STAR (tamoxifen versus raloxifene among healthy high-risk postmenopausal women) prevention trials in order to identify women at higher risk of developing breast cancer.44–46 In the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, which randomized postmenopausal women with osteoporosis to either raloxifene or placebo, women at the lowest (undetectable) estradiol levels did not seem to benefit from the use of raloxifene.47 In contrast, a nested case–cohort study within the NSABP P-1 trial showed a similar relative risk reduction across all quartiles of estradiol levels.48 However, estradiol levels overall within the NSABP P-1 cohort did not appear to be predictive of breast cancer risk, in contrast to the multiple other observational studies detailed previously, which called into question the additional value of estradiol levels in women who were already identified as high-risk by the Gail model. Therefore, it is still not clear whether circulating estrogen levels can be used to identify women for chemoprevention.
The ‘best’ hormone to assay
Although estradiol is known to have the highest binding affinity for the estrogen receptor among the circulating estrogens,49 it is still not clear which measurement would be best for breast cancer risk prediction. Although both total and free estradiol levels are associated with breast cancer risk, so are estrone and estrone sulfate, with fairly similar relative risks. It is not known whether this is due to the fact that they are correlated with each other or represent related but separate biological effects. In addition, it is not known how well circulating levels reflect exposure at the mammary tissue level. Normal mammary tissue contains enzymes that catalyze the conversion of androgens into estrogens, and estradiol levels in breast tumors have been found to be much higher than circulating levels.50,51 It is also not known whether androgens independently influence breast cancer risk or simply provide additional substrate at the tissue level for conversion into estrogens (androstenedione and estradiol can be converted directly by aromatization into estrone and estradiol, respectively). Finally, testosterone and other androgens can be more reliably assayed than estradiol, which may decrease measurement error and strengthen any observed associations.
Genetic polymorphisms in estrogen-etabolizing genes
A detailed review of all the estrogen-etabolizing genes would require a separate chapter, but clearly has been an area of intense interest. Most studies have focused on low-penetrance, high-prevalence polymorphisms in genes related both to estrogen synthesis (e.g. CYP17, CY19) and catabolism (e.g. CYP1B1, COMT). Although some relationships have been seen between individual polymorphisms and circulating estrogen levels, the associations have been modest and often have conflicting results across studies. In addition, given the multiple pathways and feedback controls inherent in the estrogen metabolism pathway, it is unlikely that a single genotype by itself would significantly influence estrogen levels.52
Relation with estrogen-receptor-negative cancers
It is increasingly recognized that breast cancer represents a heterogeneous disease rather than a single disease entity. Although different classification systems of varying complexity have been proposed, the most basic one distinguishes between hormone-receptor-positive and -negative cancers (as defined by ER and/or PR status). The few studies that have evaluated the associations with circulating hormone levels and breast cancer separately by hormone receptor status have found a stronger association with ER+/PR+ compared to ER−/PR− ones.37,41 In fact, many of the standard breast cancer risk factors with presumed hormonal mechanisms are more closely related to ER+ than ER− cancer. History of benign breast disease and family history of breast cancer which presumably operate through non-hormonal mechanisms are related to both ER+ and ER− cancers.21
SUMMARY
Both higher levels of exogenous and endogenous hormone exposure are associated with breast cancer risk. Because of the association between breast cancer and HRT, only the minimal duration of HRT use is recommended for symptom control, and it is not recommended for chronic disease management. Current research issues include the role of progestins, other types of HRT, duration of unopposed estrogen use, and characteristics of cancers that develop on HRT. Circulating sex steroids levels are associated with breast cancer risk, but multiple issues need to be addressed before they are used routinely in clinical practice. Current research issues include measurement of levels for routine clinical practice, integration with standard breast cancer risk models and genetic polymorphism data, and applicability to estrogen-receptor-negative cancers.
Practice Points
Exogenous hormones
Hormone replacement therapy with combination estrogen and progesterone increases the risk of developing invasive breast cancer. Combination HRT should only be used for the minimal duration for symptom management and should not be used for chronic disease management.
The use of conjugated equine estrogen alone for less than 7 years has not been shown to increase breast cancer risk.
Endogenous hormones
Among postmenopausal women, circulating estrogen and androgen levels have been associated with breast cancer risk, but currently clinical laboratories do not have sensitive and reliable assays to use these levels for routine breast cancer risk prediction.
Among premenopausal women, circulating androgens also appear to be associated with breast cancer risk, but the data on circulating estrogens are less clear.
Research Agenda
Exogenous hormones
Observational studies of longer durations of unopposed estrogen (>15 years) suggests that there may still be an increase in breast cancer risk.
The mechanism of the increased breast cancer risk associated with progestins is not well understood.
The effect of other formulations of HRT besides those tested in the randomized trial on breast cancer is not known.
Endogenous hormones
The “best” predictor of breast cancer among the circulating sex steroids needs to be better understood.
Research needs to be done to integrate endogenous hormone levels with current breast cancer risk prediction models and indications for chemoprevention.
Research needs to focus on non-hormonal causes of breast cancer to better understand the etiology of hormone receptor negative breast cancers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
(Note: References 4, 9, 10, 13, 29, 36 28, and 52 should have an asterisk as the most important references. I could not do so in Endnote below without disrupting the program)
- 1.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896 July 11, 1896;148(3802):104–107. [PMC free article] [PubMed] [Google Scholar]
- 2.Pike MC, Karilo M, Henderson BE, Casagrande J, Hoel D. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 3.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007 Apr 19;356(16):1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 4*.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003 Aug 9;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 5.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004 Jan 7;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Watson J, Wise L, Green J. Prescribing of hormone therapy for menopause, tibolone, and bisphosphonates in women in the UK between 1991 and 2005. Eur J Clin Pharmacol. 2007 Sep;63(9):843–849. doi: 10.1007/s00228-007-0320-6. [DOI] [PubMed] [Google Scholar]
- 7.Faber A, Bouvy ML, Loskamp L, van de Berg PB, Egberts TC, de Jong-van den Berg LT. Dramatic change in prescribing of hormone replacement therapy in The Netherlands after publication of the Million Women Study: a follow-up study. Br J Clin Pharmacol. 2005 Dec;60(6):641–647. doi: 10.1111/j.1365-2125.2005.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9*.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 10*.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003 Jun 25;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 11.McTiernan A, Martin CF, Peck JD, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: women’s health initiative randomized trial. J Natl Cancer Inst. 2005 Sep 21;97(18):1366–1376. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 12.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006 Apr 12;295(14):1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 13*.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer [see comments] [published erratum appears in Lancet 1997 Nov 15;350(9089):1484] Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 14.Executive summary. Hormone therapy. Obstet Gynecol. 2004 Oct;104(4 Suppl):1S–4S. doi: 10.1097/01.AOG.0000138807.32767.32. [DOI] [PubMed] [Google Scholar]
- 15.Preventive Services Task Force. Hormone therapy for the prevention of chronic conditions in postmenopausal women: recommendations from the U.S. Ann Intern Med. 2005 May 17;142(10):855–860. [PubMed] [Google Scholar]
- 16.Vassilopoulou-Sellin R, Cohen DS, Hortobagyi GN, et al. Estrogen replacement therapy for menopausal women with a history of breast carcinoma: results of a 5-year, prospective study. Cancer. 2002 Nov 1;95(9):1817–1826. doi: 10.1002/cncr.10913. [DOI] [PubMed] [Google Scholar]
- 17.von Schoultz E, Rutqvist LE. Menopausal hormone therapy after breast cancer: the Stockholm randomized trial. J Natl Cancer Inst. 2005 Apr 6;97(7):533–535. doi: 10.1093/jnci/dji071. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg L, Iversen OE, Rudenstam CM, et al. Increased Risk of Recurrence After Hormone Replacement Therapy in Breast Cancer Survivors. J Natl Cancer Inst. 2008;100:475–482. doi: 10.1093/jnci/djn058. [DOI] [PubMed] [Google Scholar]
- 19.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997 Aug;18(4):502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 20.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006 May 8;166(9):1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 21.Chen WY, Colditz GA. Risk factors and hormone-receptor status: epidemiology, risk-prediction models and treatment implications for breast cancer. Nat Clin Pract Oncol. 2007 Jul;4(7):415–423. doi: 10.1038/ncponc0851. [DOI] [PubMed] [Google Scholar]
- 22.Stahlberg C, Pedersen AT, Lynge E, et al. Increased risk of breast cancer following different regimens of hormone replacement therapy frequently used in Europe. Int J Cancer. 2004 May 1;109(5):721–727. doi: 10.1002/ijc.20016. [DOI] [PubMed] [Google Scholar]
- 23.Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008 Jan;107(1):103–111. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamimi RM, Hankinson SE, Chen WY, Rosner B, Colditz GA. Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch Intern Med. 2006 Jul 24;166(14):1483–1489. doi: 10.1001/archinte.166.14.1483. [DOI] [PubMed] [Google Scholar]
- 25.Ewertz M. Influence of non-contraceptive exogenous and endogenous sex hormones on breast cancer risk in Denmark. Int J Cancer. 1988 Dec 15;42(6):832–838. doi: 10.1002/ijc.2910420606. [DOI] [PubMed] [Google Scholar]
- 26.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000 Sep 15;152(6):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 27.Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC) Int J Cancer. 2004 Sep 20;111(5):762–771. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 28*.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006 Jul 12;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 29*.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003 Aug 20;95(16):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 30.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004 Feb;150(2):161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 31.Cauley JA, Lucas FL, Kuller LH, Vogt MT, Browner WS, Cummings SR. Bone mineral density and risk of breast cancer in older women: the study of osteoporotic fractures. Study of Osteoporotic Fractures Research Group [see comments] JAMA. 1996;276(17):1404–1408. [PubMed] [Google Scholar]
- 32.Zhang Y, Kiel DP, Kreger BE, et al. Bone mass and the risk of breast cancer among postmenopausal women. N Engl J Med. 1997 Feb 27;336(9):611–617. doi: 10.1056/NEJM199702273360903. [DOI] [PubMed] [Google Scholar]
- 33.Kerlikowske K, Shepherd J, Creasman J, Tice JA, Ziv E, Cummings SR. Are breast density and bone mineral density independent risk factors for breast cancer? J Natl Cancer Inst. 2005 Mar 2;97(5):368–374. doi: 10.1093/jnci/dji056. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Paris F, Sultan CS, et al. Recombinant cell ultrasensitive bioassay for measurement of estrogens in postmenopausal women. J Clin Endocrinol Metab. 2005 Mar;90(3):1407–1413. doi: 10.1210/jc.2004-0766. [DOI] [PubMed] [Google Scholar]
- 35.Lippman ME, Krueger KA, Eckert S, et al. Indicators of lifetime estrogen exposure: effect on breast cancer incidence and interaction with raloxifene therapy in the multiple outcomes of raloxifene evaluation study participants. J Clin Oncol. 2001 Jun 15;19(12):3111–3116. doi: 10.1200/JCO.2001.19.12.3111. [DOI] [PubMed] [Google Scholar]
- 36*.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002 Apr 17;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 37.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004 Dec 15;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 38.Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005 Dec;12(4):1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 39.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4(6):649–654. [PubMed] [Google Scholar]
- 40.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005 May 18;97(10):755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 41.Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006 Oct 4;98(19):1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 42.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 43.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer: does the association vary by a woman’s predicted breast cancer risk? J Clin Oncol. 2006 Apr 20;24(12):1823–1830. doi: 10.1200/JCO.2005.03.7432. [DOI] [PubMed] [Google Scholar]
- 44.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 45.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005 Nov 16;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 46.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006 Jun 21;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 47.Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002 Jan 9;287(2):216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- 48.Beattie MS, Costantino JP, Cummings SR, et al. Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP Breast Cancer Prevention Trial (P-1) J Natl Cancer Inst. 2006 Jan 18;98(2):110–115. doi: 10.1093/jnci/djj011. [DOI] [PubMed] [Google Scholar]
- 49.Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997 Mar;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 50.Blankenstein MA, van de Ven J, Maitimu-Smeele I, et al. Intratumoral levels of estrogens in breast cancer. J Steroid Biochem Mol Biol. 1999 Apr-Jun;69(1–6):293–297. doi: 10.1016/s0960-0760(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 51.Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003 Sep;86(3–5):245–253. doi: 10.1016/s0960-0760(03)00364-9. [DOI] [PubMed] [Google Scholar]
- 52*.Kendall A, Folkerd EJ, Dowsett M. Influences on circulating oestrogens in postmenopausal women: relationship with breast cancer. J Steroid Biochem Mol Biol. 2007 Feb;103(2):99–109. doi: 10.1016/j.jsbmb.2006.07.011. [DOI] [PubMed] [Google Scholar]