Abstract
Dilated human cardiomyopathy is associated with suppression of the prosurvival phosphatidylinositol-3-kinase (PI3K)/Akt and STAT3 pathways. The present study was carried out to determine if restoration of the PI3K/Akt and STAT3 activity by darbepoetin alfa improved cardiac function or reduced cardiomyocyte apoptosis in rabbit autoimmune cardiomyopathy induced by a peptide corresponding to the second extracellular loop of the β1-adrenergic receptor (β1-ECII). We found that β1-ECII immunization produced progressive LV dilation, systolic dysfunction and myocyte apoptosis as measured by TUNEL, single-stranded DNA antibody, and active caspase-3. These changes were associated with activation of p38 mitogen-activated protein kinase (MAPK), endoplasmic reticulum stress markers (GRP78 and CHOP), and increased cleavage of procaspase-12, as well as decreased phosphorylation of Akt and STAT3, and decreased Bcl2/Bax ratio. As expected, darbepoetin alfa treatment increased phosphorylation of Akt and STAT3. It also increased the myocardial expression of erythropoietin receptor which was reduced in the failing myocardium, and improved cardiac function in the β1-ECII–immunized animals. The latter was associated with reductions of myocyte apoptosis and cleaved caspase-3, as well as reversal of increased phosphorylation of p38-MAPK, increased ER stress, and decline in Bcl2/Bax ratio. The anti-apoptotic effects of darbepoetin alfa via Akt and STAT activation were also demonstrated in cultured cardiomyocytes treated with the anti-β1-ECII antibody. These effects of darbepoetin alfa in vitro were prevented by LY294002 and STAT3 peptide inhibitor. Thus, we conclude that darbepoetin alfa improves cardiac function and prevents progression of dilated cardiomyopathy probably by activating the PI3K/Akt and STAT3 pathways and reducing ER stress.
Keywords: Cardiomyocyte apoptosis, endoplasmic reticulum, MAP kinases, Akt, Bcl2, Bax
1. Introduction
Evidence has accumulated that endoplasmic reticulum (ER) stress plays an important role in many disease states including dilated cardiomyopathy. The ER is a cell organelle with interconnected network of cisternae, tubules and vesicles known to play an important role in protein translation, folding of secretary and membrane proteins, maintenance of calcium homeostasis, and production and storage of glycogen, steroids and other macromolecules (1). When the cell is exposed to obnoxious stimuli, such as hypoxia, ischemia, gene mutation, oxidative insult, or unglycosylation that increase misfolded proteins or perturb intracellular Ca2+ homeostasis in the ER, an adaptive process that couples the ER protein load with the ER protein folding capacity occurs (2). This process, known as unfolded protein response (UPR), is characterized by upregulation of ER chaperones such as glucose-regulated protein 78 (GRP78), release of activating transcription factor 6 (ATF6) to the Golgi where ATF6 is cleaved to the active p36ATF which migrates to the nucleus and binds with the ER stress response element to promote the transcription of UPR genes, and removal of the unfolded proteins to the ubiquitin proteasome for degradation. However, if ER stimuli overwhelm the capacity of UPR to remove the unfolded proteins from the ER, a maladaptive ER overload response (EOR) occurs. EOR is associated with transcriptional induction of C/EBP homologous protein (CHOP), cleavage of the ER-resident procaspase-12 to active caspase-12, and eventual programmed cell death through the activation of caspase-9 and -3 (2, 3). It has now been demonstrated that UPR and EOR are activated not only in acute myocardial ischemia/reperfusion but also in cardiac hypertrophy and failure (3–6). Dilated cardiomyopathy also has been shown to occur in transgenic mice overexpressing a mutant KDEL receptor for ER chaperones that sensitizes the cells to ER stress (7).
Our laboratory reported recently that ER stress plays an important role in cardiomyocyte apoptosis and development of dilated cardiomyopathy in rabbits immunized with a peptide corresponding to the second extracellular loop of the human β1-adrenoceptor (β1-ECII) (8). The ER stress is functionally linked to β-adrenergic receptor-mediated activation of Ca++/Calmodulin dependent protein kinase II and p-38 mitogen-activated protein (MAP) kinase (9). In addition, Akt activity was reduced in the failing myocardium, along with reductions of phosphorylation of GSK3β (9) and signal transducers and activators of transcription-3 (STAT3). Our results suggest that both activation of ER stress and suppression of the prosurvival phosphatidylinositol-3-kinase (PI3K)/Akt and STAT3 pathways are involved in β1-ECII–induced cardiomyopathy. However, little is known of the relative importance of the two cellular signaling pathways. Nor is it known if they are causally related, although activation of the PI3K/Akt pathway by insulin has been shown to reduce ER stress produced by norepinephrine in PC12 cells (10).
In this study, we proposed to investigate the effects of erythropoietin which is known to activate erythropoietin receptor (EpoR)-coupled Janus tyrosine kinase 2 (JAK2), STAT3 and the PI3K/Akt pathway (11–13), to determine if it exerts a cardioprotective effect on the β1-ECII-induced cardiomyopathy, and if activation of the PI3K/Akt and STAT3 signaling pathways is associated with reversal of ER stress in the failing myocardium. Darbepoetin alfa, a recombinant human erythropoietin analogue with a long elimination half-life (14), was chosen to allow for extended dosing intervals and less frequent administration. Darbepoetin alfa has been shown to improve exercise tolerance and clinical symptoms (15, 16), as well as systolic and diastolic cardiac function (17), in patients with chronic heart failure and anemia. The improvement in cardiac function probably is not causally related to increase in hematocrit because darbepoetin alfa increased left ventricular contractile function even at a dose that did not increase hematocrit (18). In addition, cultured neonatal rat cardiomyocytes were used to study the direct interactions between darbepoetin alfa and anti-β1-ECII antibody on the cardiomyocyte ER stress and PI3K/Akt transduction pathways, using purified monoclonal anti-β1-ECII IgG and inhibitors of PI3K and STAT3. Our results showed that erythropoietin restored EpoR expression which was depleted in the cardiomyopathic heart, and attenuated the ER stress and myocardial depression through activation of the PI3K/Akt and STAT3 prosurvival pathways.
2. Methods
This study was approved by the University of Rochester Committee on Animal Resources and conformed with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
2.1. Animal immunization and darbepoetin alfa administration
Adult New Zealand White rabbits (2.8–3.9 kg) were randomized as previously described (8) to receive either active immunization with 1 mg of a 26-amino acid β1-ECII peptide (residual 197–222; HWWRAESDDEARRCYNDPKCCDFVTNR; Bethyl Laboratories, Montgomery, TX), dissolved in 0.5 ml saline conjugated with 0.5 ml of complete or incomplete Freund's adjuvant, or sham immunization with 0.5 ml of complete or incomplete Freund’s adjuvant plus 0.5 ml saline, once a month for 6 months. To investigate if erythropoietin reversed the cardiomyopathic changes, we administered subcutaneous darbepoetin alfa (1 µg/kg) once a week to animals, beginning at end of 3 months of immunization and continuing for another 3 months, and the results were compared to animals immunized for 6 months without darbepoetin alfa treatment. This dose of darbepoetin alfa is clinically relevant and has been shown to increase red blood cells in humans. We measured blood hemoglobin in the darbepoetin alfa-treated animals once a month using a B-hemoglobin photometer (HemoCure AB Ltd., Sweden). To determine if animals developed antibodies capable of binding to darbepoetin alfa, serum samples was taken from the animals before and after 3 months of darbepoetin alfa administration, stored in −70 °C, and shipping to Amgen Inc., for antibody analysis using a Biacore surface plasmon resonance-based biosensor immunoassay.
At Month 6, the animals were lightly anesthetized with ketamine (10 m/kg, i.m.) and midazolam (0.6 mg/kg, i.m.) for transthoracic M-mode and two-dimensional echocadiography, using a 5-MHz broadband transducer and an Acuson 128XP/10c echocardiographic system (Acuson Computed Sonography, Mountain View, CA). Left ventricular end-diastolic and end-systolic dimensions were measured and left ventricular fractional shortening was calculated as follows: fractional shortening (%) = [(left ventricular end-diastolic dimension – left ventricular end-systolic dimension) X 100]/left ventricular end-diastolic dimension. The animals were also anesthetized with ketamine (35 mg/kg, i.m.), and midazolam (0.8 mg/kg, i.m.) for hemodynamic measurements of aortic and left ventricular pressures (8). The aortic and left ventricular pressures and the peak rate of rise of left ventricular pressure (dP/dt) were recorded on an IOX data acquisition and analysis system (EMKA Technologies, Falls Church, VA). Hemodynamic data were taken in triplicate over a 15–20 min resting steady state and averaged for statistical analysis. After the measurements, the animals were sacrificed and the hearts removed and weighed. Transmural left ventricular muscle blocks were embedded in paraffin blocks or stored immediately in liquid nitrogen for later analyses.
2.2. In-situ end-labeling TUNEL and anti-single-stranded DNA assays
Cardiomyocyte apoptosis was measured in the ventricular myocardium by both terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) detection using an Apoptosis Detection System (Promega, Madison, WI), and immunohistochemical staining of single-stranded DNA, using a monoclonal antibody to single-stranded DNA antibody (Millipore Chemicon International, Inc., Temecula, CA). The latter detects cells with the morphology typical of apoptosis in the early stages of apoptosis (19). Propidium iodide (Sigma-Aldrich Co., St. Louis, MO) and heavy chain myosin (Millipore Chemicon) were used to identify cardiomyocytes. The slides were visualized under an Olympus BX-FLA Reflected light fluorescence microscope (Olympus America, Center Valley, PA). Apoptotic index was calculated based on the number of TUNEL- or anti-single stranded DNA-positive cells per 10,000 cardiomyocytes.
2.3. Immunocytochemistry for erythropoietin receptors
Heart muscle paraffin sections (4 µm) were deparaffinized and hydrated for antigen retrieval. The tissue sections were incubated with polyclonal anti-EpoR antibody (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 4 °C overnight, followed by chicken anti-rabbit secondary antibody (1:500) for 1 h at room temperature. The slides were subsequently stained using an Elite ABC Vectastain kit (Vector Laboratories, Inc., Burlingame, CA), and hematoxylin to identify the nuclei.
2.4. Western immunoblotting
Left ventricular muscle was homogenized in a lysis buffer (Cell Signaling Technology, Inc., Danvers, MA), and prepared for either a whole cell lysate or ER membrane fraction by centrifugation at 12,000 × g or 100,000 × g, respectively. Protein samples (20~40 µg) were loaded onto 8~12% SDS-polyacrylamide gels and transferred electrically to PVDF membranes. Blots was then probed with the following antibodies: Monoclonal anti-caspase-12 (1:500, Sigma-Aldrich), anti-GRP78 (1:2000, BD Biosciences, San Jose, CA), anti-CHOP (1: 200, Santa Cruz), anti-phospho-Akt (Ser472), anti-Akt (1: 1000, Cell Signaling), anti-phospho-STAT3 (Ser727), anti-STAT3 (1:500 each, Cell Signaling), anti-Bcl2 (1: 1000, Santa Cruz), polyclonal anti-Bax (1:1000, Cell Signaling), anti-phospho-P38 MAPK (1:500, Cell Signaling), anti-p38 MAPK (1:1000, Cell Signaling), anti-phospho-ERK1/2 and anti-ERK1/2 (both 1:1000, Cell Signaling), and anti-EpoR antibody (1:1000, Santa Cruz). Monoclonal anti-GAPDH antibody (1:1000, Santa Cruz) was used to confirm equal protein loading. The blots were then treated with secondary antibody, and visualized using ECL detection kit (Amersham Biosciences, Piscataway, NJ). The optical density of the bands was determined using NIH 1.6 Gel image program, and the readings were normalized to a control sample in an arbitrary densitometry unit.
2.5. In vitro effects of β1-ECII antibody and darbepoetin alfa in cultured rat cardiomyocytes
Neonatal rat ventricular cardiomyocytes were cultured as described previously (8). Briefly, cardiac ventricles, taken from 1–2 days old Sprague-Dawley rat neonates, were gently minced and enzymatically dissociated repeatedly using collagenase H (Worthington Biochemical Corporation, Lakewood, NJ) in Ca- and Mg-free HBSS medium at 37 °C. Dissociated cells was then filtered through 200 µm mesh and collected by centrifugation and plated at a density of 5 × 104/cm2 on a 60-mm dish in DMEM (Cellgro, Mediatech, Inc., Herndon, VA) containing 10% (v/v) fetal bovine serum and 1% penicillin-streptomycin for 24 h. Cytosine 1-β-D-arabinofuranoside (Sigma; 10 µM) was added to retard the growth of contaminating fibroblasts.
Purified monoclonal antibody was obtained from hybridoma cells custom prepared from mice injected with the specific β1-ECII peptide (Austral Biologicals, San Ramon, CA). The monoclonal IgG is considered over 95% pure by gel electrophoresis, and recognizes the anti-β1-ECII peptide by both ELISA and Western blot analysis, with an antibody titer in excess of 1:8,000.
We first studied if the β1-ECII antibody increased TUNEL-positive cells in cultured cardiomyocytes, and if this apoptotic effect could be abolished by addition of darbepoetin alfa. We then investigated if the antiapoptotic effect of darbepoetin alfa in cultured cardiomyocytes was associated with changes in GRP78, CHOP, caspase-12, Akt and STAT3 as in the rabbit myopathic heart. Finally, to determine if the actions of darbepoetin alfa on STAT3 or Akt were functionally important in mediating its beneficial effects on ER stress and cell apoptosis, we preincubated cultured myocytes with LY294002 (10 µM, Millipore Upstate, Billerica, MA), a PI3K inhibitor, or a cell-permeable STAT3 inhibitor peptide (5 µM, Calbiochem, San Diego, CA) for 20 min. β1-ECII IgG (250 µg/mL), darbepoetin alfa (200 ng/mL), or both, were then added to cell culture medium at 37 °C for 48 h.
2.6. Statistical analysis
Results are presented as means±SEM. Experimental data were analyzed using the RS/I Research System (Bolt, Beraneck and Newman Software Products, Cambridge, MA), and SYSTAT 11 Software (Systat Software, Inc., San Jose, CA). The statistical significance of differences among the different experimental groups was analyzed by analysis of variance and post-hoc Bonferroni simultaneous confidence intervals. Differences were considered statistically significant if P< 0.05.
3. Results
3.1. Clinical characteristics
Animals tolerated the β1-ECII peptide immunization and darbepoetin alfa administration well. Five of the 16 animals treated with darbepoetin alpha developed positive binding antibodies to darbepoetin alfa with cross reactivity to epoetin alfa. None of the 12 control animals developed the binding antibodies. Blood hemoglobin, which was 12.2±0.3 and 13.2±0.4 g/dL in the Sham (n=8) and cardiomyopathic animals (n=9) at the end of Month 3, increased after darbepoetin alfa to 18.2±0.4 and 17.2±0.5 g/dL, respectively, at Month 4. However, it did not increase further despite continuing administration, and was not statistically different between the two darbepoetin alfa-treated groups of animals (17.0±1.3 vs. 18.6±1.2 g/dL) at Month 6.
Table 1 shows that body weight, heart rate, mean aortic pressure, and heart weight did not differ significantly among the 4 experimental groups at Month 6. However, cardiomyopathy was evidenced in the β1-ECII–immunized animals by the increases in left ventricular end-diastolic dimension and pressure, and decreases in left ventricular dP/dt and fractional shortening. Administration of darbepoetin alfa which had no effects in Sham animals reduced left ventricular dilation and filling pressure, and increased left ventricular dP/dt and fractional shortening in the cardiomyopathic animals. Anti-darbepoetin antibody was positive in two of the β1-ECII–immunized rabbits treated with darbepoetin alfa. When the two animals were removed from analyses, the mean left ventricular dP/dt and fractional shortening increased slightly with no changes in conclusion.
Table 1.
Effects of darbepoetin alfa on hemodynamics and cardiac function in β1-ECII–induced cardiomyopathy
| Sham Immunization | β1-ECII Immunization | |||
|---|---|---|---|---|
| Control | Darbepoetin alfa | β1-ECII | β1-ECII + darbepoetin alfa | |
| N | 10 | 7/8 | 13 | 7/8 |
| Body weight (kg) | 3.9±0.1 | 3.6±0.1 | 4.0±0.2 | 4.2±0.2 |
| Heart rate (bpm) | 266±9 | 294±14 | 224±13 | 266±24 |
| Mean aortic pressure (mmHg) | 99±4 | 101±7 | 88±5 | 100±9 |
| Left ventricular | ||||
| Weight (g) | 5.3±0.2 | 5.1±0.3 | 5.3±0.1 | 5.5±0.2 |
| EDP (mmHg) | 5.1±0.5 | 6.0±0.5 | 16.2±1.9* | 9.2±1.9† |
| dP/dt (mmHg/s) | 5131±251 | 5626±444 | 2961±300* | 4468±538† |
| EDD (mm) | 15.5±0.1 | 15.8±0.2 | 17.6±0.2* | 16.7±0.2*† |
| FS (%) | 36.9±1.3 | 39.0±1.7 | 27.9±0.6* | 32.7±1.5*† |
Values are means±SEM. N = number of animals in each group.
P< 0.05, compared to Control Sham immunization group.
P< 0.05 compared to Control β1-ECII immunization group.
3.2. Myocyte apoptosis
Cardiomyocyte apoptosis occurred in the failing myocardium of the β1-ECII–immunized rabbits, as evidenced by the increases of TUNEL- and anti-single-stranded DNA-positive cardiomyocytes (Table 2). In addition, the cell apoptosis in the cardiomyopathic heart was associated with a 6-fold increase in cleaved caspase-3. Administration of darbepoetin alfa to the Sham animals produced no effect in the number of TUNEL positive cells, anti-single-stranded DNA, or cleaved caspase-3. However, when administered to the β1-ECII–immunized animals, it markedly reduced the increase in apoptosis index and caspase-3 activation.
Table 2.
Effects of darbepoetin alfa on cardiomyocyte apoptosis index and active caspase 3 in β1-ECII–induced cardiomyopathy
| Sham Immunization | β1-ECII Immunization | |||
|---|---|---|---|---|
| Control | Darbepoetin alfa | β1-ECII | β1-ECII + darbepoetin alfa | |
| Apoptosis index | ||||
| TUNEL assay | 12.4±2.2 | 9.9±0.9 | 40.3±4.3* | 20.1±1.4*† |
| Anti-ss DNA | 12.8±3.5 | 9.5±2.6 | 89.8±15.5* | 34.0±5.8*† |
| Active caspase 3 | 1.00±0.06 | 0.98±0.05 | 6.20±0.29* | 1.16±0.11† |
Values are means±SEM. N=6–8 animals in each group. Antiapoptosis index is defined by the number of TUNEL– or anti-single stranded DNA (ss DNA) antibody–positive cells per 105 cardiomyocytes. Active caspase-3 protein expression was given in arbitrary densitometry units, with results normalized to Control Sham-immunization group.
P< 0.05, compared to Control Sham immunization group.
P< 0.05 compared to Control β1-ECII immunization group.
3.3. Myocardial erythropoietin receptors
EpoR protein expression was markedly reduced in the cardiomyopathic myocardium, as evidenced by immunohistochemistry and Western blotting (Figure 1). Administration of darbepoetin alfa increased EpoR expression in Sham animals. It also reduced the loss of EpoR in β1-ECII–immunized animals.
Figure 1.
Changes in erythropoietin receptor (EpoR) expression in left ventricular myocardium of rabbits following β1-ECII immunization, darbepoetin alfa administration, or both. Panel A. Representative EpoR expression by immunohistochemistry. Panel B. EpoR protein expression by Western blots. N=6 animals in each group. Representative immunoblots for EpoR and GAPDH are shown on the left to the bar graph. Bar denote SEM. *P<0.05, compared to Sham immunization, † P<0.05, compared to β1-ECII immunization, and ‡ P<0.05, compared to darbepoetin alfa alone.
3.4. Akt/STAT/Bcl2 signaling pathways
Figure 2 shows that myocardial phospho-Akt and phospho-STAT3 were decreased in the β1-ECII–immunized rabbit hearts, and that these change were abolished by the addition of darbepoetin alfa to the β1-ECII–immunized animals. In contrast, neither total Akt nor STAT3 changed significantly in the cardiomyopathic heart. Also shown on this figure are the reductions of phospho-Akt/Akt and phospho-STAT3/STAT3 ratios, and concomitant increase of Bax and decrease of Bcl2 in the cardiomyopathic heart, and the reversal of these changes by darbepoetin alfa administration. The ratio of Bcl2/Bax decreased in the cardiomyopathic heart (0.26±0.06, P<0.001, compared to the Sham control [1.00±0.09]) and this decrease was greatly minimized by administration of darbepoetin-treatment in the β1-ECII–immunized animals (0.68±0.10, P<0.001, compared to control β1-ECII–immunized animals).
Figure 2.
Effects of darbepoetin alfa on left ventricular myocardial phosphorylated Akt (p-Akt), phosphorylated STAT3 (p-STAT3), Bax and Bcl-2 in the Sham– and β1-ECII–immunized rabbits. Representative immunoblots are shown on the left to the bar graphs. p-Akt and p-STAT3 are expressed as ratios of total Akt and total STAT3, respectively, in the bar graphs. N=6 animals in each group. Bars denote SEM. *P<0.05, compared to Sham immunization, and † P<0.05, compared to β1-ECII immunization.
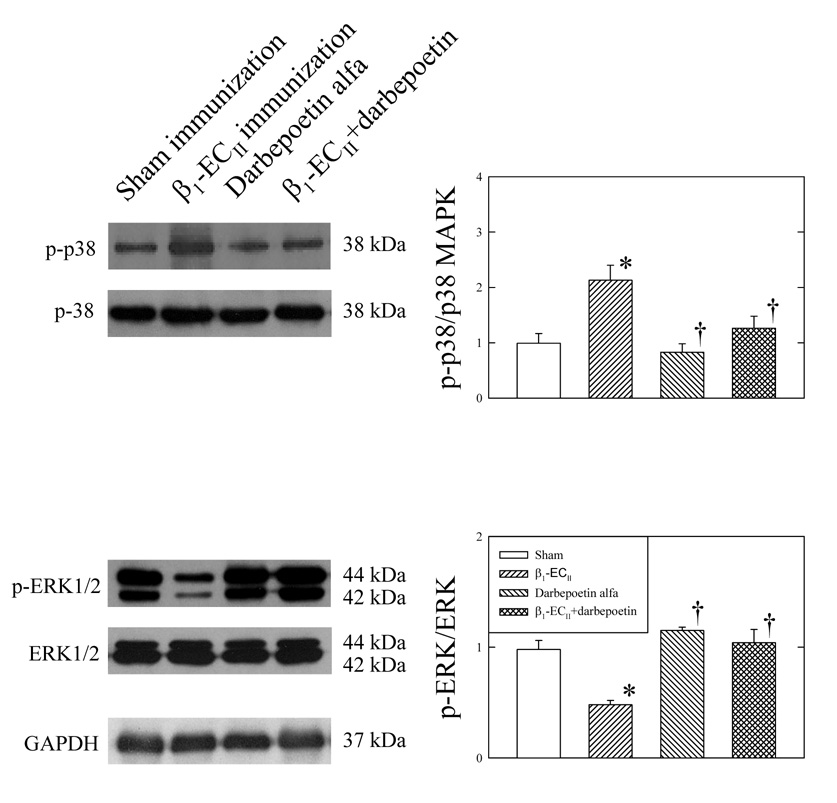
3.5. MAP kinases and ER stress
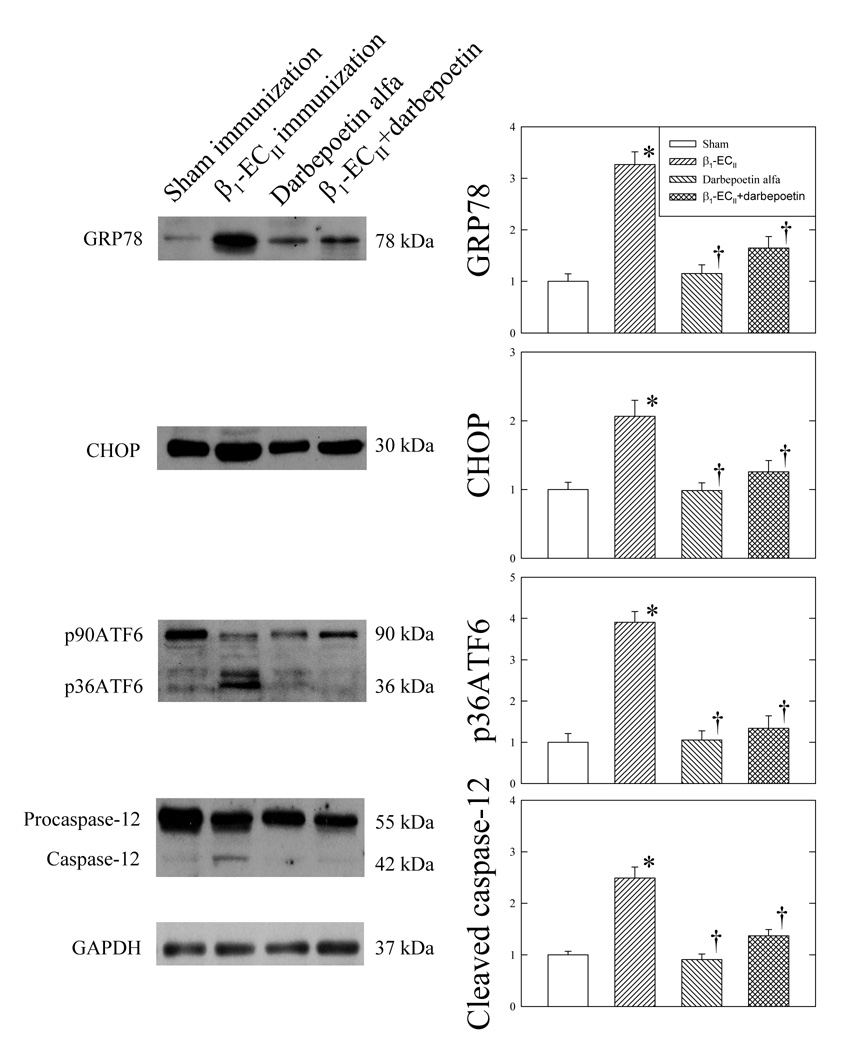
Cardiomyopathic myocardium exhibited increased protein expression of phospho-p38 MAPK and decrease in phospho-ERK1/2 (Figure 3), while neither total p38 MAPK nor total ERK1/2 changed significantly. Figure 4 shows that β1-ECII immunization also increased GRP78, and CHOP protein expression, and cleavage of p90ATF6 to p36ATF6, and procaspase-12 to active caspase-12. Administration of darbepoetin alfa produced no appreciable changes in any of the parameters in Sham rabbits (Figure 3 and Figure 4). However, in the β1-ECII–immunized rabbits, administration of darbepoetin alfa led to not only reversal of the increases in MAP kinases and ER proteins, but also attenuation of the cleavage of caspase-12 in the cardiomyopathic heart.
Figure 3.
Effects of darbepoetin alfa on left ventricular myocardial p-38 MAP kinase and ERK1/2 in the Sham– and β1-ECII–immunized rabbits. Representative immunoblots are shown on the left to the bar graphs. Phosphorylated p38-MAPK (p-38) and phosphorylated ERK1/2 (p-ERK) were expressed as ratios of total p38-MAPK and total ERK1/2, respectively, in the bar graphs. N=6 animals in each group. Bars denote SEM. *P< 0.05, compared to Sham immunization. † P< 0.05 compared to β1-ECII immunization.
Figure 4.
Effects of darbepoetin alfa on left ventricular myocardial GRP78, CHOP, ATF6, and caspase-12 in the Sham– and β1-ECII–immunized rabbits. Representative immunoblots are shown on the left to the bar graphs. N=6 animals in each group. Bar denote SEM. *P<0.05, compared to Sham immunization, and † P<0.05, compared to β1-ECII immunization.
3.6. Cultured neonatal rat cardiomyocyte studies
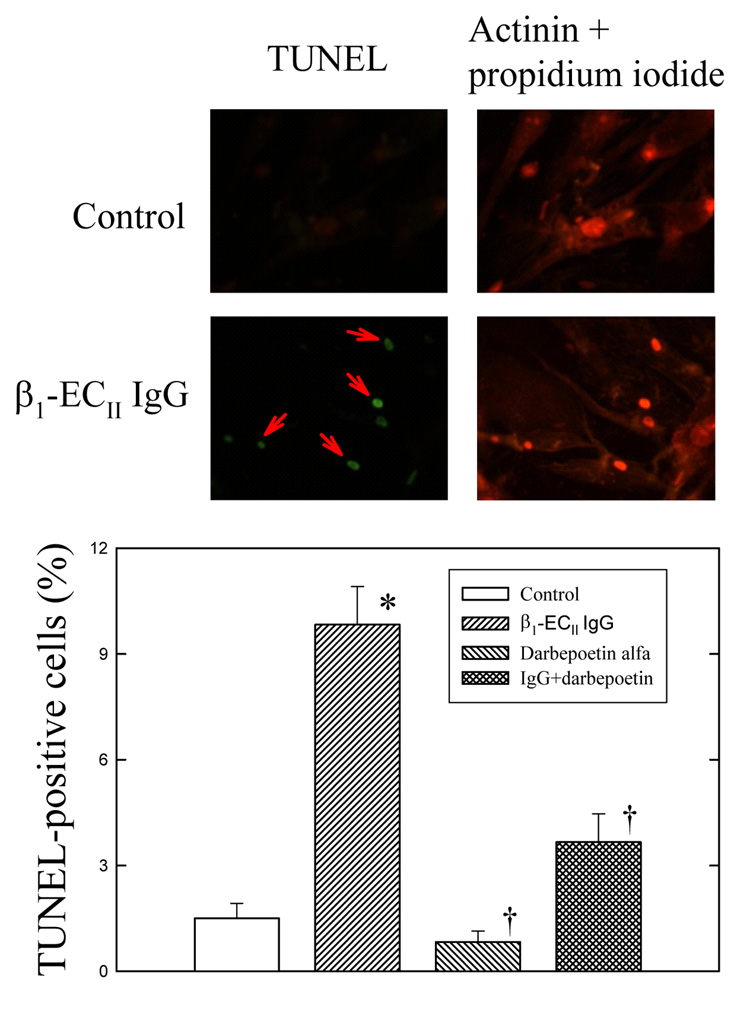
Monoclonal β1-ECII IgG produced dose-dependent cardiomyocyte apoptosis. Figure 5 shows that the number of TUNEL-positive cardiomyocytes increased after 48 h incubation of β1-ECII IgG. β1-ECII IgG also increased GRP78, CHOP proteins and cleaved caspase-12 in cultured cardiomyocytes (Figure 6A). These effects were associated with reductions in phospho-Akt and phospho-STAT3 (Figure 6B), with no changes in total Akt or STAT3. Addition of darbepoetin alfa, which had no direct effects on the number of TUNEL-positive cells when given alone (Figure 5), reversed the reductions in phospho-Akt and phospho-STAT3 produced by β1-ECII IgG (Figure 6B) and significantly reduced the β1-ECII IgG–induced increases of TUNEL-positive cells (Figure 5), GRP78, CHOP, and cleaved caspase-12 (Figure 6A).
Figure 5.
Effects of β1-ECII IgG and darbepoetin alfa on cell apoptosis in cultured neonatal rat cardiomyocytes. Representative micrographs are given on the top panel showing the increase of TUNEL-positive cardiomyocytes (indicated by red arrows) after addition of β1-ECII IgG, and the cardioprotective effect of darbepoetin alfa in the β1-ECII IgG–treated cardiomyocytes (left panel). Cardiomyocytes are identified by the actinin and propidium iodide stains (right panel). The bottom panel shows the statistical difference in cardiomyocyte apoptosis among the groups. N=6 in each group. Bar denote SEM. *P<0.05, compared to Control, and † P<0.05, compared to β1-ECII IgG.
Figure 6.
Effects of β1-ECII IgG, darbepoetin alfa, LY294002 and STAT3 inhibitor peptide on ER stress markers GRP78, CHOP, and cleaved caspase-12 (Figure 6A), and phosphorylated Akt (p-Akt) and phosphorylated STAT3 (p-STAT) (Figure 6B) in cultured neonatal rat cardiomyocytes. Representative immunoblots are shown below the corresponding bar graphs. N=6 in each group. Bar denote SEM. *P<0.05, compared to Control. † P<0.05, compared to β1-ECII IgG. ‡ P<0.05, compared to β1-ECII IgG plus darbepoetin alfa.
Figure 6B also shows that LY294002 reduced the effects of darbepoetin alfa on phospho-Akt and phospho-STAT3, but addition of STAT3 inhibitor peptide reduced only the expression of phospho-STAT3 in the β1-ECII IgG–treated cardiomyocytes. The findings suggest that STAT3 phosphorylation occurs after activation of PI3K/Akt. Also, because addition of LY294002 and STAT3 inhibitor peptide reversed the effects of darbepoetin alfa on GRP78, CHOP and cleaved caspase-12 produced by β1-ECII IgG (Figure 6A), the beneficial effect of darbepoetin alfa on ER stress was mediated at least in part via the activation of the PI3K/Akt pathway.
4. Discussion
Autoimmune cardiomyopathy induced by β1-ECII immunization has been extensively studied in experimental animals (8, 20–22). The anti-β1-ECII antibody also has been shown to increase intracellular calcium transients, activate the CaMKII and p-38 MAPK signaling and ER stress and induce myocyte apoptosis directly in cultured cardiomyocytes (9, 23). In this study, we add that phosphorylation of myocardial Akt and STAT3 was reduced in the β1-ECII–immunized rabbits, which is consistent with the decreased expression of tyrosine phosphorylation of JAK and STAT3 in patients with end-staged dilated cardiomyopathy (24). In addition, we provide a novel finding that the PI3K/Akt and STAT3 signal transduction pathways important in the pathophysiology of cardiomyopathy, as darbepoetin alfa which stimulates the PI3K/Akt and STAT3 systems was shown to reduce ER stress, myocyte apoptosis and cardiodepression in the β1-ECII–induced cardiomyopathy.
Erythropoietin and EpoR are essential for full expression of tissue protective effects of erythropoietin. Our present study showed that EpoR expression was reduced in the failing myocardium. The findings suggest that the failing myocardium with EpoR downregulation may be hyporesponsive to endogenous erythropoietin, and that this can be restored by exogenous erythropoietin. Among other mechanisms, receptor internalization and degradation are known to occur for receptor downregulation (25). EpoR is present in the ER. A possibility exists that EpoR is reduced in the failing myocardium because of the ER stress–mediated unfolded protein response which prevents full maturation and translocation of EpoR to the plasma membrane. We have shown that such a mechanism is responsible for the reduction of norepinephrine transporter in PC12 cells exposed to oxidative stress (26). However, it is not known whether gene expression for EpoR is also reduced in the failing myocardium. The restoration of EpoR by darbepoetin alfa in cardiomyopathy probably is related to the improvement in ER function, but other independent cellular signaling mechanisms cannot be excluded.
Erythropoietin appears to have separate functional domains for its hematotrophic and nonhematopoietic tissue protective function (27, 28). Indeed, a carbamylated derivative of erythropoietin without the erythropoiesis-stimulating property has been developed to study the nonhematopoietic tissue-protective action of erythropoietin (29, 30). Studies have also shown that the actions of erythropoietin rely on its binding to EpoR, as erythropoietin produced no anti-cardiac remodeling effect in ischemic myocardium in transgene-rescue EpoR null mutant mice which lack EpoR in nonhematopoietic tissues (31). The immediate mechanism of action of erythropoietin probably involves EpoR dimerization and tyrosine phosphorylation of the receptor by Janus activating kinase 2 (JAK2) (11). JAKs are then activated and cause tyrosine phosphorylation of the Src homology 2 domains of various proteins such as STATs (32), PI3K (12), and MAP kinases (33). In the JAK family, JAK1 and JAK2 are preferentially activated by erythropoietin and confer a cardioprotective action (33–36). The STAT family consists of 7 members. Once they are phosphorylated by JAKs, STAT proteins homodimerize or heterodimerize and translocate to the nucleus where they bind to specific promoter sequences of STAT-responsive genes and activate STAT-specific transcription (37). It is known that STAT1 activation is proapoptotic in cardiac ischemia, while STAT3 activation antagonizes the apoptosis-promoting effects of STAT1 and serves as an intrinsic protective pathway in the cells (36, 38). STAT3 has been shown to play an important role in the signal transduction cascade in the heart for both ischemic preconditioning (33) and ischemic postconditioning (39). Studies have also shown that the age-related loss of ischemic postconditioning is probably caused by the reduced levels of STAT3 in the aged hearts (39). Our present study demonstrates an antiapoptotic effect of STAT3 in autoimmune cardiomyopathy. We also observed in preliminary studies that STAT1 phosphorylation was increased in the β1-ECII-immunized heart, but its role in the pathophysiology of autoimmune cardiomyopathy remains to be investigated. It has been stated that the opposing effects of apoptotic STAT1 and antiapoptotic STAT3 in the cells probably are related to the competition of STAT1 and STAT3 for the same DNA binding domain and phosphorylation sites for JAK and MAPK (40). A complex competitive interaction also exists between the STATs and MAPKs for JAK2. Haq et al. (41) showed that ERK inhibitor reduced the phosphorylation of both STAT1 and STAT3 by JAK2, but p38 MAPK inhibitor affected only the STAT1, not STAT3 phosphorylation.
Akt is a serine threonine kinase important in cell proliferation and survival. Akt is phosphorylated following PI3K activation by a number of cytokines and growth factors such as insulin, erythropoietin, and vascular endothelial growth factor. Our results show that Akt phosphorylation was reduced in the failing myocardium of β1-ECII–immunized rabbits, but that it was reduced by a direct action of β1-ECII IgG in cultured cardiomyocytes. We also showed that deactivation of Akt was functionally linked to decreased phosphorylation of STAT3 in the diseased heart and cultured cardiomyocytes, and that the antiapoptotic effect of darbepoetin alfa was associated with increases in both phospho-Akt and phospho-STAT3. Results of our studies with PI3K and STAT3 inhibitors also indicate that STAT3 activation is an event distal to Akt phosphorylation. These findings are consistent with that the morphine-induced STAT3 phosphorylation in myocardial ischemia/reperfusion was abolished by wortmannin, a PI3K/Akt inhibitor (34), and that acetylation of STAT3 at Lys-685, which is critical for STAT3 activation, by leukemia inhibitory factor or interlukin-6 (IL-6) was suppressed by LY294002 or a dominant negative Akt (42). However, our results do not exclude the possibility that PI3K/Akt and STAT3 may act in parallel. It has been reported that three independent signal transduction pathways (i.e., JAK/STAT, SH2 domain-containing tyrosine phosphatase (SHP2)/Ras/ERK, and PI3K/Akt), are involved in the IL-6 induced activation of glycoprotein 130 receptor system (43). Furthermore, although granulocyte colony-stimulating factor phosphorylated both STAT3 and Akt in infarcted myocardium, its beneficial effects on anti-cardiomyocyte degeneration and antifibrosis were abolished only by parthenolide, a STAT3 inhibitor, and not by wortmannin, whereas the vascular effects of granulocyte colony-stimulating factor were affected only by wortmannin (44). The findings suggest that STAT3 and PI3K/Akt may work separately but act in concert to exert their cardioprotective effects.
Similar to our present study, LY294002 has been shown to reduce the anti-apoptotic effect of erythropoietin on neonatal rat cardiomyocytes exposed to hypoxia (45). The molecular mechanism downstream of Akt, however, is unclear, but probably involves Pim-1 because its overexpression has been shown to inhibit cardiomyocyte apoptosis and increase Bcl2 and Bcl-XL proteins (46). In our study, the Bcl2/Bax ratio which was reduced in the failing heart was reversed by darbepoetin alfa. The changes in Bcl proteins probably also are a result of the binding of Akt/STAT3 to the Bcl-related genes in the nucleus (47).
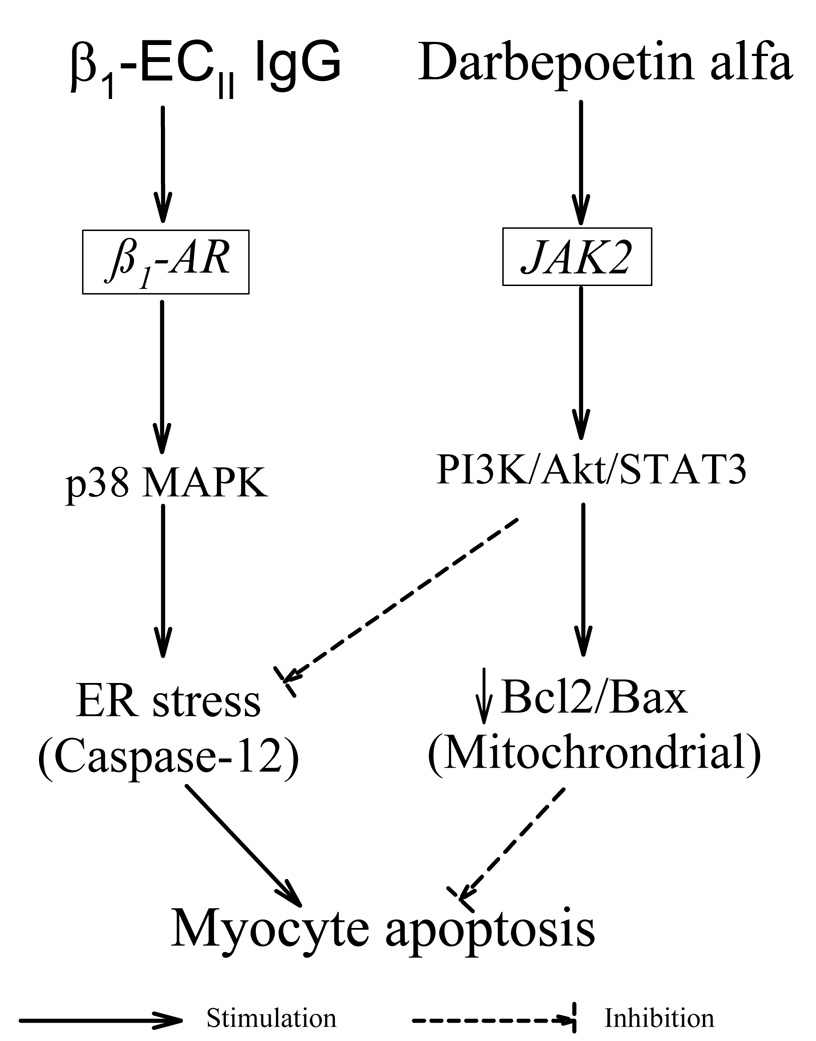
Our present study shows that the PI3K/Akt and STAT3 systems and the ER are closely linked in cardiomyopathy (Figure 7). The ER stress in autoimmune cardiomyopathy and in β1-ECII–treated cardiomyocytes was associated with a reduction in phospho-Akt, while the reversal of ER stress by darbepoetin alfa was accompanied by normalization of phospho-Akt. The importance of the PI3K/Akt pathway in the mediation of the cytoprotective effect of darbepoetin alfa in β1-ECII IgG–induced ER stress was further confirmed by the use of LY294002 and STAT3 inhibitor peptide. These findings are analogous to our prior findings that the ER stressors thapsigargin and norepinephrine reduced phospho-Akt in PC12 cells, and that Akt activation by insulin conferred the PC12 cells resistance to ER-induced cell apoptosis (10). Furthermore, the ER stress–induced CHOP expression is increased when the PI3K/Akt pathway is inactivated (48). However, the molecular mechanism linking the PI3K/Akt signaling pathway to the ER has not been fully explored. Nevertheless, evidence emerges that the Bcl2 homolog domain-3-only protein bimakalim (Bim), a known substrate of activated Akt, may play an important role in the initiation of ER stress-induced apoptosis. Bim, which is sequestered by the prosurvival Bcl protein in healthy cells, may be freed when the level of Bcl protein is reduced, and translocates to the ER to activate caspase-12 (49) and ER stress-induced apoptosis by both protein phosphatase 2A-mediated dephosphorylation and CHOP-mediated direct transcription induction (50). The downward shift of the Bcl2/Bax ratio in our autoimmune cardiomyopathic hearts is expected not only to activate the intrinsic mitochondrial death pathway by increasing mitochondrial membrane permeability and cytochrome C release, but also favor the translocation of Bim to the ER causing activation of the ER-resident caspase-12. On the other hand, when Akt activity was increased by darbepoetin alfa, Bcl2/Bax ratio increased and antiapoptotic effects ensued.
Figure 7.
A schematic diagram showing the proposed mode of action of darbepoetin alfa on activation of the prosurvival JAK/PI3K/Akt pathway, and inhibition of the ER-stress–mediated cardiomyocyte apoptosis induced by β1-ECII IgG.
Not unexpectedly, we found that at the doses administered, the human erythropoietin analogue darbepoetin alfa increased blood hemoglobin and induced anti-darbepoetin alfa antibodies in some rabbits. However, the cardiac protective effects of erythropoietin have been shown to be independent of its action on red blood cell production (28–30). The carbamylated derivative of erythropoietin, devoid of the erythropoietic effects, retains the cardiac protective effects of erythropoietin in both myocardial ischemia-reperfusion injury (51) and chronic myocardial infarction (30).
In summary, darbepoetin alfa exerts an important antiapoptotic cardiac protective effect in autoimmue cardiomyopathy. This effect appears to be mediated via its action of the EpoR-19 coupled PI3K/Akt and STAT transduction signaling pathways, which leads to not only a shift of BCl2/Bax ratio in favor of cell survival but also a reduction of ER stress-induced caspase-12–mediated cell apoptosis. These actions on PI3K/Akt probably are linked also to the effects of erythropoietin on reduction of inflammatory cytokines and oxidative stress (52), and increased cardiac vascular endothelial growth factor expression and capillary growth (53, 54). However, since the cardiac protective effects of erythropoietin are evidenced in isolated perfused hearts and cultured cardiomyocytes, neovascularization is not likely to be a predominant factor. Finally, further studies should be carried out to investigate the beneficial non-hematopoietic cardioprotectve effects of erythropoietin in human cardiomyopathy. Efforts should also be directed toward discovery of novel nonerythropoietic derivatives with selective antiapoptotic actions via the PI3K/Akt and STAT3 cytoprotective mechanisms without effects on thrombosis or promotion of cancerous growth.
Acknowledgments
We gratefully acknowledge the excellent technical support provided by Dr. Xujing Xie, Ms. Megan Simeone and Ms. Robin Stuart-Buttles.
The study was supported in part by National Heart, Lung, and Blood Institute Grant HL-68151, and a research grant from Amgen, Inc. There is no relationship that may be perceived as an actual or possible conflict of interest with the content of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Intracellular compartments and protein sorting. 5th ed. New York: Garland Science; 2008. Molecular Biology of the Cell. Chapter 12; pp. 695–748. [Google Scholar]
- 2.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glembotski CC. The role of the unfolded protein response in the heart. J Mol Cell Cardiol. 2008;44:453–459. doi: 10.1016/j.yjmcc.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 5.Tsukamoto O, Minamino T, Okada K-i, Shintani Y, Takashima S, Kato H, Liao Y, Okazaki H, Asai M, Hirata A, Fujita M, Asano Y, Yamazaki S, Asanuma H, Hori M, Kitakaze M. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun. 2006;340:1125–1133. doi: 10.1016/j.bbrc.2005.12.120. [DOI] [PubMed] [Google Scholar]
- 6.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 7.Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004;24:8007–8017. doi: 10.1128/MCB.24.18.8007-8017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao W, Fukuoka S, Iwai C, Liu J, Sharma VK, Sh.eu S-S, Fu M, Liang C-s. Cardiomyocyte apoptosis in autoimmune cardiomyopathy: mediated via endoplasmic reticulum stress and exaggerated by norepinephrine. Am J Physiol Heart Circ Physiol. 2007;293:H1636–H1645. doi: 10.1152/ajpheart.01377.2006. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Mao W, Iwai C, Fukuoka S, Vulapalli R, Huang H, Sharma V, Sheu S-S, Fu M, Liang C-s. Adoptive passive transfer of rabbit β-adrenoceptor peptide immune cardiomyopathy into the Rag2−/− mouse: participation of the ER stress. J Mol Cell Cardiol. 2008;44:304–314. doi: 10.1016/j.yjmcc.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao W, Iwai C, Keng PC, Vulapalli R, Liang C-s. Norepinephrine-induced oxidative stress causes PC-12 cell apoptosis by both endoplasmic reticulum stress and mitochondrial intrinsic pathway: inhibition of phosphatidylinositol 3-kinase survival pathway. Am J Physiol Cell Physiol. 2006;290:C1373–C1384. doi: 10.1152/ajpcell.00369.2005. [DOI] [PubMed] [Google Scholar]
- 11.Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Thie JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 12.Haseyama Y, Sawada K-i, Oda A, Koizumi K, Takano H, Tarumi T, Nishio M, Handa M, Koike T. Phosphatidylinositol 3-kinase is involved in the protection of primary cultured human erythroid precursor cells from apoptosis. Blood. 1999;94:1568–1577. [PubMed] [Google Scholar]
- 13.Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Khai MC, Maruyama R, Ogino A, Minatoguchi S, Fujiwara T, Fujiwara H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–543. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- 14.Ohls RK, Dai A. Long-acting erythropoietin: clinical studies and potential uses in neonates. Clin Perinatol. 2004;31:77–89. doi: 10.1016/j.clp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Anker SD, Szachniewicz J, Okonko D, Ledwidge M, Zymlinski R, Ryan E, Wasserman SM, Baker N, Rosser D, Rosen SD, Poole-Wilson PA, Banasiak W, Coats AJS, McDonald K. Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure. A randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2007;49:753–762. doi: 10.1016/j.jacc.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Ghali JK, Anand IS, Abraham WT, Fonarow GC, Greenberg B, Krum H, Massie BM, Wasserman SM, Trotman M-L, Sun Y, Knusel B Armstrong P on behalf of the Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation. 2008;117:526–535. doi: 10.1161/CIRCULATIONAHA.107.698514. [DOI] [PubMed] [Google Scholar]
- 17.Parissis JT, Kourea K, Panou F, Farmakis D, Paraskevaidis I, Ikonomidis I, Filippatos G, Kremastinos DT. Effects of darbepoetin α on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am Heart J. 2008;155:751.e1–751.e7. doi: 10.1016/j.ahj.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Lipsic E, Westenbrink BD, van der Meer P, van der Harst P, Voors AA, van Veldhuisen DJ, Schoemaker RG, van Gilst WH. Low-dose erythropoietin improves cardiac function in experimental heart failure without increasing haematocrit. Eur J Heart Fail. 2008;10:22–29. doi: 10.1016/j.ejheart.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L. Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res. 1996;226:387–397. doi: 10.1006/excr.1996.0240. [DOI] [PubMed] [Google Scholar]
- 20.Matsui S, Fu MLX, Katsuda S, Hayase M, Yamaguchi N, Teraoka K, Kurihara T, Takekoshi N, Murakami E, Hoebeke J, Hjalmarson Å. Peptides derived from cardiovascular G-protein-coupled receptors induce morphological cardiomyopathic changes in immunize rabbits. J Mol Cell Cardiol. 1997;29:641–655. doi: 10.1006/jmcc.1996.0307. [DOI] [PubMed] [Google Scholar]
- 21.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohse MJ. Direct evidence for a β1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buvall L, Täng MS, Isic A, Andersson B, Fu M. Antibodies against the β1-adrenergic receptor induce progressive development of cardiomyopathy. J Mol Cell Cardiol. 2007;42:1001–1007. doi: 10.1016/j.yjmcc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Staudt Y, Mobin R, Fu M, Felix SB, Kühn JP, Staudt A. β1–Adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur J Pharmacol. 2003;466:1–6. doi: 10.1016/s0014-2999(03)01431-6. [DOI] [PubMed] [Google Scholar]
- 24.Podewski, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.cir.0000057545.82749.ff. [DOI] [PubMed] [Google Scholar]
- 25.Walrafen P, Verdier F, Kadri Z, Chrétien S, Lacombe C, Mayeux P. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood. 2005;105:600–608. doi: 10.1182/blood-2004-03-1216. [DOI] [PubMed] [Google Scholar]
- 26.Mao W, Iwai C, Qin F, Liang C-s. Norepinephrine induces endoplasmic reticulum stress and downregulation of norepinephrine transporter density in PC-12 cells via oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H2381–H2389. doi: 10.1152/ajpheart.00904.2004. [DOI] [PubMed] [Google Scholar]
- 27.Campana WM, Misasi R, O’Brien JS. Identification of a neurotrophic sequence in erythropoietin. Development. 1998;1:235–241. doi: 10.3892/ijmm.1.1.235. [DOI] [PubMed] [Google Scholar]
- 28.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fraelli M, Latini R, Xie Q-w, Smart J, Su-Rick C-j, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common β–subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbyraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie Q-w, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 30.Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- 31.Ueda K, Takano H, Hasegawa H, Komuro I. Erythropoietin prevents cardiac remodeling after myocardial infarction through erythropoietin receptor–induced signaling pathways in cardiomyocytes. J Mol Cell Cardiol. 2008;44:439. (abstract) [Google Scholar]
- 32.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui ALF, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. Embo J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafiee P, Shi Y, Su J, Pritchard KA, Jr, Tweddell JS, Baker JE. Erythropoietin protects the infant heart against ischemia-reperfusion injury by triggering multiple signaling pathways. Basic Res Cardiol. 2005;100:187–197. doi: 10.1007/s00395-004-0508-1. [DOI] [PubMed] [Google Scholar]
- 34.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3β. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 35.Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, Yamauchi-Takihara K. Activation of JAK/STAT transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res. 2000;47:787–805. doi: 10.1016/s0008-6363(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 36.Xuan Y-T, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA. 2001;98:9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darnell JE., Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 38.Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, Knight RA, Latchman DS. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem. 2000;275:10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- 39.Boengler K, Buechert A, Heinen Y, Roeske C, Hifiker-Kleiner D, Heusch G, Schultz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Blenis J, Li H-C, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 41.Haq R, Halupa A, Beattie BK, Mason JM, Zanke BW, Barber DL. Regulation of erythropoietin-induced STAT serine phosphorylation by distinct mitogen-activated protein kinases. J Biol Chem. 2002;277:17359–17366. doi: 10.1074/jbc.M201842200. [DOI] [PubMed] [Google Scholar]
- 42.Ohbayashi N, Ikeda O, Taira N, Yamamoto Y, Muromoto R, Sekine Y, Sugiyama K, Honjoh T, Matsuda T. LIF- and IL-6 induced acetylation of STAT3 at Lys-685 through PI3K/Akt activation. Biol Pharm Bull. 2007;30:1860–1864. doi: 10.1248/bpb.30.1860. [DOI] [PubMed] [Google Scholar]
- 43.Fischer P, Hilfiker-Kleiner D. Role of gp130-mediated signaling pathways in the heart and its impact on potential therapeutic aspects. Br J Pharmacol. 2008;153:S414–S427. doi: 10.1038/bjp.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada H, Takemura G, Li Y, Ohno T, Li L, Maruyama R, Esaki M, Miyata S, Kanamori H, Ogno A, Nakagawa M, Minaoguchi S, Fujiwar T, Fujiwara H. Effect of a long term treatment with a low dose granulocyte colony-stimulating factor on postinfarction process in the heart. J Cell Mol Med. 2008 Feb 24; doi: 10.1111/j.1582-4934.2008.00294.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–994. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- 46.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmnauel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, House SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 47.Bromberg J, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanes C, Darnell JE., Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 48.Hyoda K, Hosoi T, Horie N, Okuma Y, Ozawa K, Nomura Y. PI3K-Akt inactivation induced CHOP expression in endoplasmic reticulum-stressed cells. Biochem Biophys Res Commun. 2006;340:286–290. doi: 10.1016/j.bbrc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Morishima N, Nakanishi K, Tsuchiya K, Shibata T, Seiwa E. Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress–induced apoptosis. J Biol Chem. 2004;279:50375–50381. doi: 10.1074/jbc.M408493200. [DOI] [PubMed] [Google Scholar]
- 50.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akra S, Bouillet P, Strasser A. ER stress apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 51.Fiordaliso F, Chimenti S, Staszewsky L, Bai A, Carlo E, Cuccovillo I, Doni M, Mengozzi M, Tonelli R, Ghezzi P, Coleman T, Brines M, Cerami A, Latini R. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T, Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006;71:684–694. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 54.Asaumi Y, Kagaya Y, Takeda M, Yamaguchi N, Tada H, Ito K, Ohta J, Shiroto T, Shirato K, Minegishi N, Shimokawa H. Protective role of endogenous erythropoietin system in nonhematopoietic cells against pressure overload–induced left ventricular dysfunction in mice. Circulation. 2007;115:222–232. doi: 10.1161/CIRCULATIONAHA.106.659037. [DOI] [PubMed] [Google Scholar]