Abstract
Chronic Granulomatous Disease (CGD) is characterized by defects in the superoxide producing enzyme NADPH oxidase causing phagocytes to improperly clear invading pathogens. Here we report findings of a late presenting 16 year old female with X-linked CGD. The patient presented with community-acquired pneumonia, but symptoms persisted for 2 weeks during triple antimicrobial coverage. Cultures revealed Aspergillus fumigatus which was resolved through aggressive voriconazole treatment. Neutrophil studies revealed NADPH oxidase activity and flavocytochrome b558 levels that were 4–8% of controls and suggested carrier status of the mother. We found a null mutation in the CYBB gene (c.252insAG) predicting an aberrant gp91phox protein (p.Cys85fsX23) in the heterozygous state. Methylation analysis demonstrated extremely skewed X chromosome inactivation favoring the maternally inherited defective gene. In conclusion, a novel mutation in the CYBB gene and an extremely skewed X-inactivation event resulted in the rare expression of the CGD phenotype in a carrier female.
Keywords: CGD, X-inactivation, flavocytochrome b558, neutrophil, carrier, gp91phox, CYBB, NADPH oxidase, primary immunodeficiency
Introduction
Chronic granulomatous disease (CGD) is a genetic syndrome characterized by a dysfunction of the respiratory burst, which is necessary to kill certain phagocytized pathogens [1; 2]. This disease occurs in approximately 1 in 250,000 people worldwide [3]. The neutrophils of CGD patients fail to adequately generate oxygen-dependent mechanisms to kill microorganisms. The respiratory burst response is mediated by a variety of reactive oxygen species that both directly and indirectly kill phagocytized bacteria and fungi [4; 5]. Some organisms take advantage of the compromised host, including catalase-positive organisms such as Staphylococcus aureus, as well as Burkholderia cepacia, Serratia species, Pseudomonas species, Nocardia species, Candida species and perhaps most fatally, Aspergillus species [3]. Classically, CGD is associated with recurrent, severe infections of the skin, lungs, liver, spleen and lymph nodes. As the name implies, granulomas are another feature of this disease. These granulomas occur principally in the skin, gastrointestinal and genitourinary tracts, leading to obstructive lesions that may need to be treated with steroids or surgery [1; 3; 6].
The fundamental defect in CGD lies in the NADPH oxidase, the enzyme complex responsible for initiating the respiratory burst. Defects in four of the NADPH oxidase components allow for the various phenotypic expressions of CGD. The four components of the NAPDH complex implicated in CGD are gp91phox, p22phox, p47phox and p67phox. Two of these proteins, gp91phox and p22phox, make up the membrane-associated flavocytochrome b558. The gp91phox subunit is thought to contain all the electron transport machinery of the oxidase as well as the NADPH and FAD binding sites [7]. The p22phox subunit promotes gp91phox maturation through heterodimer formation, and acts as a binding site for the cytosolic components of the NADPH oxidase. The p47phox and p67phox proteins are located in the cytosol [8] and translocate to the membrane and bind the flavocytochrome upon neutrophil stimulation. This causes a conformational change in the latter allowing for the production of superoxide anions through the electron transport from NADPH to molecular oxygen [9]. Defects in any of the four subunits can manifest as CGD. Thus, CGD patients can be phenotypically similar but genetically heterogeneous depending on which NADPH oxidase component is defective. The most common form of CGD is X-linked recessive resulting from a mutation of the CYBB gene encoding for gp91phox. CYBB is located within the Xp21.1 band region and accounts for approximately 70% of all cases of CGD. The remaining 30% of cases are caused by defects in the other three proteins, all of which are inherited in an autosomal recessive manner [3; 10].
The membrane bound flavocytochrome forms a stable heterodimer of gp91phox and p22phox during its maturation and a defect in one membrane subunit leading to a loss of expression is reflected in the loss of expression of the other. Thus, patients with CGD caused by a gene defect leading to the lack of protein expression in either the X-linked CYBB gene (gp91phox) or the CYBA gene (p22phox) will not express either protein [2; 11; 12]. In contrast, a deficiency resulting in the loss of expression of any one of the cytosolic subunits p47phox or p67phox can be identified directly by Western blot and/or flow cytometry [13; 14; 15].
Other components of the NADPH complex include p40phox and Rac 1 or 2. There have been no reported cases of CGD caused by p40phox gene defects. There is a single reported case of a child with a Rac2 mutation leading to clinically significant phagocytic cell dysfunction. The child’s phenotype more closely resembled Leukocyte Adhesion Defect in its clinical picture than it did CGD [16; 17].
The X-linked recessive form of CGD is by far the most common, resulting in a greater number of affected males. However, there have been reports of affected females with the diagnosis of X-linked CGD [18; 19; 20; 21; 22; 23; 24; 25] attributed to skewed X-inactivation. According to the Lyon hypothesis of X chromosome inactivation, "either one of the two X's may be inactivated in different cells of the same animal, and the inactivation occurs early in development"[26]. Generally this is a random process of inactivation, with an expected ratio of 50:50, although in reality the ratio follows a Gaussian distribution [27]. Thus, female carriers of the X-linked form of CGD should be mosaics, with approximately half of their cells expressing the healthy normal X chromosome, and the remaining cells expressing the mutated, disease carrying X chromosome. However, certain X chromosome mutations seem to influence the ratio to favor the inactivation of the disease carrying X chromosome: a nonrandom X-inactivation with selective advantage. There are numerous hypotheses as to why nonrandom X-inactivation occurs, however there is no one prevailing theory [28].
Here we report a unique patient with CGD having the following features: a female with the X-linked form of the disease secondary to extremely skewed X chromosome inactivation; a novel, previously unreported mutation of the CYBB gene that codes for gp91phox protein; and a late diagnosis at age sixteen. There is no family history of CGD; however the patient's mother and sister are confirmed carriers of the disease, exhibiting the classic mosaic of flavocytochrome expressing neutrophils.
Materials and Methods
Patient
The patient is a 16 year old female who presented to our institution with a four week history of cough and high fevers. She initially presented to her primary care physician with fever, chills, myalgia and cough. Prior to referral, chest radiograph revealed diffuse bilateral infiltrates; the diagnosis of community acquired pneumonia was made and she was discharged on oral antibiotics. Her symptoms persisted for two weeks, at which time she was admitted to a local hospital, with computed tomography of chest demonstrating diffuse bilateral infiltrates, significantly worse than previous imaging. Bronchoscopy was performed and coverage broadened to include antifungal and anti-tuberculosis agents, and the patient was transferred to our institution. Bronchoalveolar lavage fluid and sputum grew colonies of Aspergillus fumigatus, despite ten days of antifungal therapy. The patient was treated aggressively for six weeks with voriconazole and improved rapidly. Follow up computed tomography of the chest demonstrated significant improvement of her infiltrates. Initial nitroblue tetrazolium (NBT) test was inconclusive because of technical issues and a dihydrorhodamine-123 (DHR) assay showed impaired oxidative burst capacity (9.68% DHR-positive cells in the unstimulated condition vs. 13.8% DHR-positive cells in the PMA stimulated condition). Subsequently, the patient has been placed on daily oral prophylaxis with itraconazole and trimethoprim/sulfamethoxazole. She also receives interferon gamma - 1b (ACTIMMUNE, InterMune, Inc.) injected subcutaneously three times a week.
Past history was significant for no major illnesses or hospitalization, but did include recurrent axillary "boils," beginning at 7 months of age, with worsening over the past two years. The site of her lesions did express purulent material and she would occasionally develop fever. The lesions required lancing and oral antibiotics about once a year. She denied any history of previous pneumonia, other deep/soft tissue, urinary tract, central nervous system, hematologic, gastrointestinal, sinopulmonary or mucocutaneous infections. Her immunizations are all current for age.
Family medical history reveals no history of primary immunodeficiency. The patient's mother developed rectal cancer at about age 40 and was diagnosed with Crohn's disease sometime later. Two older half siblings (different fathers), one male and one female, are both healthy. The mother has six full siblings, and their histories are significant for diabetes mellitus type two and rectal cancer. A maternal female first cousin has systemic lupus erythematosus, requiring a renal transplant.
Materials
Phosphate buffered saline solution (PBS) without Ca2+ + Mg2+ and Hanks’ Balanced Salt Solution (HBSS) were obtained from Life Technologies (Carlsbad, CA). Sterile H2O was obtained from Baxter Healthcare Corporation (Deerfield, IL). Luminol was obtained from SERVA (Heidelberg, Germany). Nicotamide adenine dinucleotide phosphate (NADPH) was obtained from Boehringer-Ingelheim (Petersburg, VA). 1,2-Didecanoyl-sn-Glycero-3-Phosphate (10:0 PA) and 1-Oleoyl-2-Acetoyl-sn-Glycerol (OAG) were obtained from Avanti® Polar Lipids Inc (Alabaster, AL). Arachidonic acid (AA) was obtained from NuChekPrep (Elysian, MN). All other chemicals were obtained from Sigma® (St. Louis, MO). Antibodies were generously donated by Dr. Algirdas Jesaitis (Montana State University (Bozeman, MT); 54.1-gp91phox, 44.1-p22phox [29]), Dr. Tom Leto (NIH (Bethesda, MD); Anti-p47phox, p67phox, and p40phox [30]), and Prof. Nakamura (Nagasaki University (Nagasaki, Japan); 7D5 [31]). The anti-Rac antibody (Cat# 05-389) was purchased from Upstate Biotechnology (Lake Placid, NY). Secondary antibodies were from BD Biosciences and Pierce.
Low Endotoxin Neutrophil Isolation
Neutrophils were isolated as previously described [32; 33] with modifications. All reagents used in this procedure were sterile and of the lowest endotoxin content commercially available. In brief, heparinized venous blood was obtained from the patient, the patient’s mother and sister, and normal subjects following informed consent, using a protocol approved by the Wake Forest Institutional Review Board. Erythrocytes were removed by gravity sedimentation over Isolymph (Gallard-Schlesinger, Plainview, NY). The leukocyte-rich suspension was centrifuged over Isolymph and contaminating erythrocytes were removed by hypotonic lysis. Neutrophils were resuspended in HBSS at 1×108 cells/mL.
Luminol-Enhanced Chemiluminescence
The neutrophil respiratory burst was monitored by luminol-dependent chemiluminescence (CL) [34]. Isolated neutrophils (5×104 cells/mL) in HBSS were incubated with 20µM luminol in a white 96-well plate (Greiner, Lake Mary, FL). Stimulus [1µM N-formyl-methionyl-leucyl-phenylalanine (fMLP), 10µM dihydrocytochalasin B (dCB) followed by 1µM fMLP, 0.05mg/mL opsonized zymosan (OPZ) prepared as previously described [35], or 100nM phorbol myristate acetate (PMA)] was added and light emission was monitored continuously at 25°C for the indicated time period in a MicroLumat Plus LB 96 V luminometer (Berthold Technologies, Oak Ridge, TN). Data are expressed as relative light units (RLUs).
Subcellular Fractionation of Neutrophils
Isolated neutrophils in HBSS were treated with 1.71mM diisopropyl fluorophosphate, resuspended at 2×108 cells/mL in ice-cold sonication buffer (50mM Tris, pH 7.4, 11% sucrose, 100mM NaCl, 2mM EGTA, 2mM EDTA, 25mM NaF, 10µg/mL leupeptin, 10µg/mL pepstatin, 1µg/mL aprotinin, and 1mM PMSF) and sonicated on ice to ~ 90% cell breakage. Membrane and cytosolic fraction proteins were separated on a 15/40% discontinuous sucrose gradient and stored at −70°C until needed [36]. Protein concentrations were determined by the Bradford assay [37].
Cell-Free NADPH Oxidase Assay
Cell-free NADPH oxidase assays were performed as previously described [38]. In brief, reaction mixtures (50mM NaxPO4, pH 7.0, 1mM EGTA, 5mM MgCl2, 10µM FAD, 0.1mM cytochrome c, 1µM guanosine 5’-o-(3-thiotriphosphate), 0.5µg membrane protein, 12.5µg cytosolic protein) were incubated with or without 0.05mg/mL superoxide dismutase and activated with the addition of lipid (30µM OAG + 30µM 10:0 PA or 25µM AA) for 60 min at 25°C. NADPH (0.2mM) was added to induce superoxide production and the rate of superoxide dismutase-inhibitable cytochrome c reduction was measured continuously at 550nm on a UV-2401 PC spectrophotometer (Shimadzu, Kyoto, Japan). NADPH oxidase activity was determined from linear slopes using the cytochrome c extinction coefficient of 21mM−1cm−1 [39]. Data are expressed as nmol of superoxide produced/min/mg of membrane protein.
Western Blot Analysis
Protein samples were prepared in Laemmli sample buffer and separated using SDS-PAGE on 7%, 10%, or 14% polyacrylamide gels [40]. Proteins were transferred to nitrocellulose and Western blot analysis was performed as described [41]. In brief, blots were blocked for 1 hr with 5% milk in TBST (10mM Tris, 100mM NaCl, and 0.1% Tween 20), washed, and then incubated with appropriate primary antibody for 2 hr at room temperature. Blots were then washed in TBST followed by HRP-conjugated secondary antibody incubation (~1 hr) and detection by CL.
Flow Cytometry Analysis
Flow cytometric analysis was performed as previously described [42] but with modifications. Isolated neutrophils (5×106 cells/mL) were suspended in cold PBS with 0.1% gelatin and incubated with mouse IgG or mAb 7D5 (0.03mg/mL) for 30 min on ice. Cells were washed 3x with cold PBS/gelatin before adding FITC-GAM (1:100) to all conditions. Cells were incubated for 30 min on ice under foil, then washed 3 times and resuspended in 1mL of PBS/gelatin. Flavocytochrome b558 expressing cells were counted using a BD FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). Data are expressed as number of events versus fluorescent intensity.
X Chromosome Inactivation Analysis
DNA was extracted from peripheral blood and isolated neutrophils from the patient and her mother using a standard commercial protocol (Qiagen Inc., Valencia, CA). X chromosome inactivation analysis using the HUMARA gene followed a previously published protocol with slight modifications [43]. Briefly, 1µg of DNA from peripheral blood and neutrophils from the patient and mother, along with appropriate controls, were digested with HhaI (20U/ul) and HpaII (10U/ul) (New England BioLabs Inc., Ipswich, MA) at 37°C overnight followed by enzymatic inactivation by heating at 95°C for 10 min. Separate PCR amplification was performed using 100ng of digested and undigested DNA from all samples using primers specific for the methylation regions of the HUMARA gene. Fluorescently labeled PCR products were denatured for 97°C for 3 min then analyzed by capillary gel electrophoresis using an ABI 3100 Genetic Analyzer and ABI GeneScan software (Applied Biosystems, Foster City, CA). X chromosome inactivation ratios were determined using the peak heights for the various PCR products using the following formula:
CYBB Genetic Analysis
Bi-directional sequence of all 13 coding exons of the X-linked CYBB gene and their intron/exon boundaries were obtained and analyzed by GeneDx DNA diagnostic services (Gaithersburg, MD).
Results
Evidence for neutrophil dysfunction
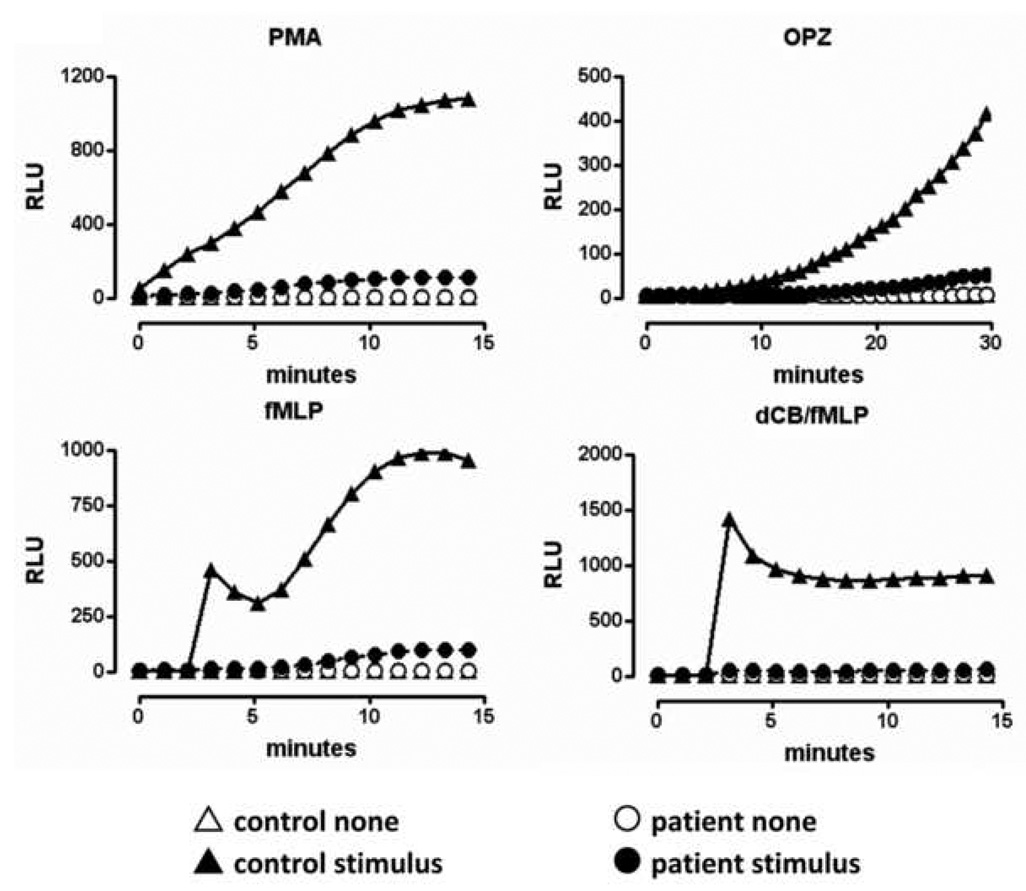
To determine if the patient had neutrophils capable of producing a measurable respiratory burst, the luminol-enhanced CL assay was performed on isolated neutrophils from both the patient and a control volunteer. We used three different stimuli (PMA, OPZ, fMLP) because each one is known to result in NADPH oxidase activation through different pathways [44]. As shown in Fig. 1, the neutrophils of the patient showed a markedly reduced respiratory burst response in response to all stimuli tested compared to control cells. By using the area under the curve for the patient’s respiratory burst response to each of the stimuli tested, we determined the percentage of functional response compared to that of the control subject. The patient’s functional response as compared to that of control cells was as follows: PMA = 7.94%, OPZ = 7.78%, fMLP = 7.58% and dCB/fMLP = 4.72%. This low response was confirmed using a whole cell cytochrome c reduction assay (data not shown). This small, but measurable respiratory burst response suggests some NADPH oxidase activity persists in the patient’s cells, but that this activity is insufficient for effective resistance to Aspergillus infection.
Figure 1. Evidence for neutrophil dysfunction.
Isolated neutrophils in HBSS/0.1% gelatin from the patient (circles) and a control donor (triangles) were incubated in the presence of 20µM luminol. The indicated stimulus (solid symbols) or buffer (None, open symbols) was added either directly (100nM PMA or 0.05mg/ml OPZ) or after established baseline readings (1µM fMLP or 10µM dCB + 1µM fMLP). Relative light units (RLU) were recorded continuously for 15 or 60 min depending on the stimulus. All stimuli were tested in duplicate wells and the patient’s cells were examined on two different occasions. Data shown are from one experiment.
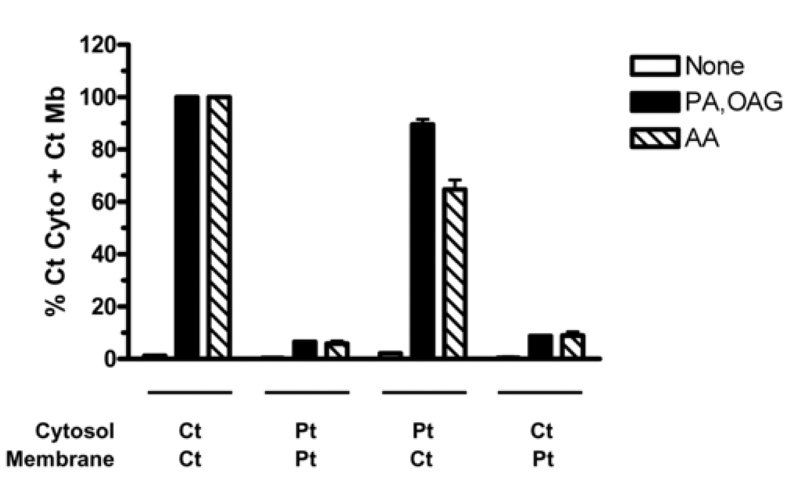
In the intact cell, it is difficult to discern which component of the oxidase is functionally deficient because of the numerous subunits that are needed for proper complex formation. By using a cell-free system [45], we can distinguish whether the defect is in the membrane-associated or cytosolic components of NADPH oxidase. Membrane and cytosolic fractions were isolated from patient and control neutrophils and mixed in the presence of cell-free activators (10:0 PA+OAG, AA) to induce NADPH oxidase activity. As shown in Fig. 2, the mixture of control membrane and cytosolic fractions resulted in normal superoxide production. Mixing only patient fractions resulted in low oxidase activity, indicating that the patient has an NADPH oxidase defect. Mixing the patient’s cytosolic fraction with the control membrane fraction showed normal superoxide production in the system, indicating that NADPH oxidase components in the cytosol were not defective. In contrast, only low levels of oxidase activity were obtained when the control cytosolic fraction was mixed with the patient’s membrane fraction. This reduction indicates a deficiency in the patient’s membrane-bound oxidase components, e.g. flavocytochrome b558.
Figure 2. Membrane-bound components of the patient’s NADPH oxidase are functionally deficient.
Cytosolic and membrane fractions were prepared from the neutrophils of the patient (Pt) and a normal healthy control donor (Ct). The fractions (12.5µg cytosol and 0.5µg membrane) were mixed in the indicated combinations and incubated as described in the Methods with either water (empty bars), 100µM 10:0 PA + 100µM OAG (solid bars) or 25µM AA (striped bars) for 60min at 25°C. NADPH (0.2mM) was added and the reduction of cytochrome c was measured at 550nm. Data are expressed as a percentage of control cytosol plus control membrane (Ct Cyto + Ct Mb) for each activator. Specific activities for the control fractions (Ct Cyto + Ct Mb) expressed as nmol O2−/min/mg memb were: None = 43±9, PA/OAG = 3397±23, AA = 1745±196 (Mean ± SEM, n=3).
Flavocytochrome b558 expression is diminished in the neutrophils of the patient
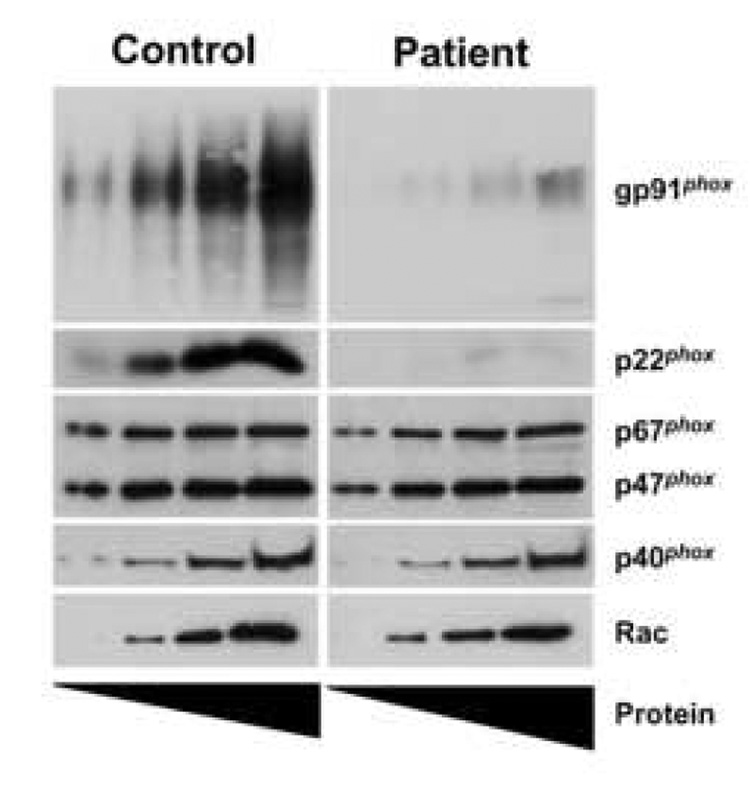
In a complementary approach, the levels of NADPH oxidase components in the isolated cytosolic and membrane fractions were assessed by Western blot analysis. Fig. 3 shows normal expression of the cytosolic oxidase components p47phox, p67phox, p40phox and Rac in the patient’s neutrophils. In contrast, the amounts of flavocytochrome b558 subunits p22phox and gp91phox were markedly diminished, but detectable. This low level of flavocytochrome b558 likely accounts for the markedly decreased respiratory burst in the patient’s neutrophils.
Figure 3. The patient expresses low levels of gp91phox and p22phox.
Increasing amounts of isolated neutrophil cytosolic and membrane fractions (1, 2.5, 5, 10µg for gp91phox; 5, 10, 20, 40µg for all other components) from both a control subject (left) and the patient (right) were prepared in Laemmli sample buffer, subjected to SDS-PAGE and transferred to nitrocellulose. Western blot analysis was performed on the membrane fractions for gp91phox and p22phox. Cytosolic fractions were probed for p67phox, p47phox, p40phox and Rac. The blots are representative of two experiments.
The patient has two distinct populations of neutrophils expressing different levels of flavocytochrome b558
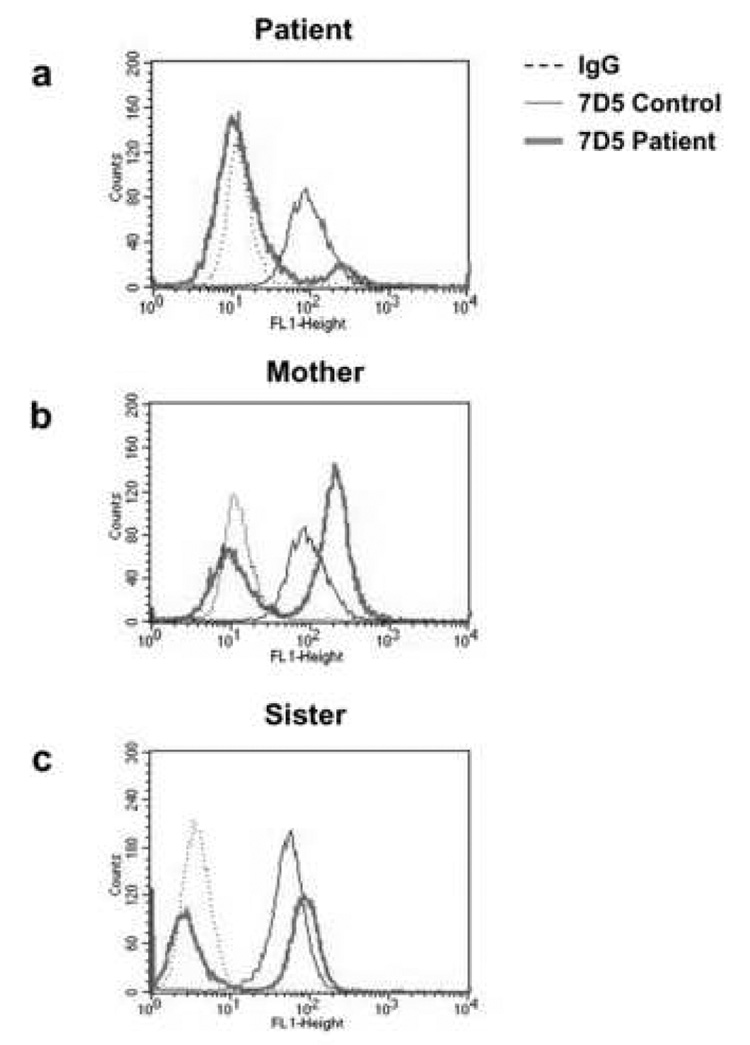
The previous experiments do not identify which of the flavocytochrome subunits is affected, since each subunit requires expression of the other subunit for stability. If the defect is in the CYBA gene, this gene would be expressed in all of the patient’s neutrophils and assessment of flavocytochrome b558 expression by flow cytometry would reveal a single population of cells. In contrast, if the defect is in the CYBB gene, the defective gene would be expressed in a sub-population of the patient’s neutrophils. We assessed the level of flavocytochrome b558 expression in individual cells by flow cytometry, using the monoclonal antibody 7D5, which recognizes an extracellular epitope of native gp91phox [31]. Fig. 4 shows that a single population of flavocytochrome b558 – expressing neutrophils was observed for the normal control donors. In contrast, neutrophils from the patient, her mother, and her sister exhibited two populations of neutrophils with respect to flavocytochrome b558 expression. The flavocytochrome b558-negative neutrophils (92.9% of total) in the patient’s sample (Figure 4a) greatly outnumbered the flavocytochrome b558-positive (7.1% of total) cells. In contrast, the distribution of cells between these two populations in the mother and sister showed a higher (61.9% and 52.1% respectively) percentage of flavocytochrome b558 positive neutrophils. The intact cell respiratory burst activity elicited in the neutrophils of the mother and sister were not different from that of normal, healthy control cells (data not shown) despite the presence of a population of flavocytochrome b558-negative cells. The dual populations in the neutrophils from the patient, her mother, and her sister are indicative of an XCGD carrier [46; 47], suggesting the presence of a coding defect in the CYBB gene in one population of cells.
Figure 4. The patient, her mother, and sister are X-CGD carriers.
Isolated neutrophils (5×106 cells/mL) from a control donor (thin solid line) or the indicated subject (thick solid line, 4a, Patient; 4b, Mother; 4c, Sister) were suspended in cold PBS/0.1% gelatin and stained with either irrelevant IgG (dashed lines) or mAb 7D5 (solid lines). Cells were then washed and incubated with FITC-goat anti-mouse secondary Ab and analyzed in a flow cytometer. Data are expressed as number of cells versus fluorescent intensity and are representative of at least three experiments.
The patient demonstrates extremely skewed X-inactivation
The flow cytometry results suggest the possibility that the patient has skewed X-inactivation. We used the HUMARA assay to assess the extent of X chromosome inactivation in the patient and the mother. This assay is based on methylation-sensitive, HhaI and HpaII restriction sites located on the HUMARA (human androgen-receptor gene) locus on the X chromosome. In DNA obtained from whole blood and isolated neutrophils, both the normal control and the patient’s mother demonstrated normal X-inactivation while the patient’s DNA showed a markedly skewed X-inactivation event (Table 1). These results are in general agreement with the results from the flow cytometric assay (Figure 4). If we assume that the methylation of the HUMARA allele is indicative for the methylation of CYBB on the same X chromosome, then an extremely skewed X-inactivation event (normal range 50 to 89%; skewed 90 to 100% [48]), favoring expression of the defective CYBB gene, would explain why this patient displays the X-CGD phenotype when she appears to be an XCGD carrier.
Table 1. X chromosome inactivation is skewed and mirrors the flavocytochrome b558 expression levels the patient and her mother.
The HUMARA gene was used to determine X chromosome inactivation described in the Methods. Results are shown on the left. %XA/B represents the overall methylation of chromosome A or B for the indicated cell type (results shown from one blood draw). The percent of total neutrophils that express (%Pos) or do not express (%Neg) flavocytochrome b558 based on flow cytometric analysis are shown on the right (n≥3).
| Methylated DNA |
Flavocytochrome b558 Expression | |||||
|---|---|---|---|---|---|---|
| Whole Blood | Neutrophils | |||||
| % XA | % XB | % XA | % XB | % Neg | % Pos | |
| Control | 59.7 | 40.3 | 64.0 | 36.0 | 0.0 | 100.0 |
| Mother | 55.1 | 44.9 | 61.5 | 39.5 | 39.1 | 61.9 |
| Patient | 95.7 | 4.3 | 96.4 | 3.6 | 92.9 | 7.1 |
The patient has a heterozygous single base change in intron 3 of the CYBB gene
The identification of the patient as a CGD carrier with a skewed X-inactivation event localized the gene defect to the CYBB gene. In order to determine the precise mutation in CYBB, blood samples were sent to GeneDx, Inc. (Gaithersburg, MD) for analysis. Bi-directional sequencing of all 13 coding exons of the X-linked CYBB gene and their intron/exon boundaries revealed a heterozygous single base change of A→G at the 3’ end of intron 3. A schematic representation of the projected effects of this mutation is shown in Fig. 5. The mutation analysis suggests a potential disruption of the wild type splice site between intron 3 and exon 4, which would result in a more favorable splice site two bases upstream. This alternate splice site allows for a 2bp insertion of intronic sequence into the mRNA coding for gp91phox. The intronic insertion is predicted to result in a frame shift and a new, premature stop signal 23 codons downstream of the mutation. Consequently, a non-functional, severely truncated (~107 amino acids) form of the protein would be expressed, which is likely to be unstable, accounting for the lack of expression of flavocytochrome b558 in one population of the neutrophils from the patient, her mother and her sister.
Figure 5. The patient’s mutation is predicted to result in the expression of a severely truncated and nonfunctional form of gp91phox.
The CYBB sequence results and the proposed translations of exon 4 in gp91phox are shown for the wild type (upper panel) and the patient (lower panel). Wild type intronic DNA is in lower case and the wild type exonic DNA is in upper case lettering. The coding DNA for both wild type and patient sequence is boxed and its corresponding translation is shown below the sequence. The bolded lettering indicates the patient’s variation from the wild type sequence. The ‘ag’ intronic insertion resulting from the patient’s mutation leads to mistranslation and a premature stop signal 23 codons downstream of Ala 84.
Discussion
Based on this patient’s presentation with persistent and recurring Aspergillus infections and suspicious laboratory results for the neutrophil respiratory burst, we used a comprehensive series of laboratory assays to explore the possible presence of CGD. We demonstrated that the patient’s neutrophils showed a pronounced decrease in the respiratory burst in response to physiological stimuli that are known to activate the respiratory burst response through a variety of signaling pathways [44]. This was the first indication that the patient has a defect within the NADPH oxidase components themselves and not in the signaling pathways leading to activation of the functional response. Subsequent experiments localized the defect first to the membrane fraction and then to a deficiency of flavocytochrome b558. Flow cytometry results revealed that the patient is an X-CGD carrier, with only 7–8% of her cells expressing flavocytochrome b558.
X-CGD consists of three subtypes; X91° (gp91phox not expressed), X91− (low gp91phox expression), and X91+ (gp91phox expressed but not functional) [49]. Our results indicate that this patient is the X91− genotype, caused by extremely skewed X-inactivation of the chromosome carrying the normal gene. The mutation we identified predicts expression of a truncated protein, which appears to be unstable. Previous studies indicate that proper gp91phox expression is needed for p22phox expression [50]. This explains why the cells expressing the defective gene do not express either gp91phox or p22phox.
Carrier-status with a CYBB gene defect being secondary to an extremely skewed X-inactivation event is rare (<2% of all reported CGD cases), but has been observed [18; 25; 51; 52; 53; 54]. Initial presentation of the CGD phenotype typically occurs in childhood, however several cases involving CGD carriers appear later in life, suggesting the possibility of a shift in X-inactivation with age [18; 53; 54]. While our patient has had a history of chronic infections, her latest infection was more severe, consistent with the possibility that her skewing might have shifted. As with other reported cases of X-inactivation within CGD, our patient exhibits a small but significant respiratory burst response (Fig 1). While this was not enough to overcome her infection alone, it may be possible to enhance this response in the future if breakthroughs in controlling the regulation of X-inactivation can be accomplished, allowing for a purposeful shift favoring the expression of her paternal X chromosome. Consequently, an analysis of X-inactivation over time can be useful in the assessment of females with atypical presentations of CGD.
X-inactivation is controlled through DNA methylation. DNA methylation has been linked to numerous disease states such as cancer, imprinting disorders and birth defects, as well as autoimmune disease [55]. Regulation of DNA methylation is poorly understood, but is thought to involve the expression of the Xist locus as well as long interspersed nuclear elements (LINEs) clustered around this loci [28]. While these factors were not explored in our case, they could play a part in the disease development of our patient and would be useful to examine in the future.
The unique mutation in the CYBB gene found in this patient is a single base change of (A→G) two bases upstream of the wild type splice site at the intron 3/exon 4 border. This mutation is thought to create a more favorable splice site. The resulting exon is predicted to not only translate incorrectly but lead to a prematurely truncated gene product due to the creation of a new stop codon downstream (Fig 5). Splice site mutations in the CYBB gene leading to X-CGD consist of less then 20% of all the known CYBB mutations [56]. Furthermore, this particular mutation has not been reported in any known CGD database.
In conclusion, we have described the manifestation of Chronic Granulomatous Disease in a 16 year old female XCGD carrier secondary to an extremely skewed X-inactivation event. To our knowledge, this is the first report of a patient carrying a splice site mutation in the CYBB gene in conjunction with skewing X-inactivation. The patient is on prophylactic antibiotics and doing well.
Acknowledgements
This work was supported by grants (L.C. McPhail, P.I.) from the March of Dimes Research Foundation (MOD 1-FY02-191) and the National Institutes of Health (R01 AI 22564). E. M. Lewis was supported by a training grant from the National Institutes of Health (T32 GM-063485), MOD 1-FY02-191, and a graduate fellowship from the Wake Forest University Graduate School of Arts and Sciences. We are appreciative of the clinical expertise and contributions of Dr. Mary Fontana-Penn, Departments of Medicine and Pediatrics, Wake Forest University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamani NR, Infante AJ. Chronic granulomatous disease and other disorders of neutrophil function. Clin Rev Allergy Immunol. 2000;19:141–156. doi: 10.1385/CRIAI:19:2:141. [DOI] [PubMed] [Google Scholar]
- 2.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Johnston RB, Jr, Keele BB, Jr, Misra HP, Lehmeyer JE, Webb LS, Baehner RL, RaJagopalan KV. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975;55:1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J Allergy Clin Immunol. 2004;113:620–626. doi: 10.1016/j.jaci.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Barton LL, Moussa SL, Villar RG, Hulett RL. Gastrointestinal complications of chronic granulomatous disease: case report and literature review. Clin Pediatr (Phila) 1998;37:231–236. doi: 10.1177/000992289803700403. [DOI] [PubMed] [Google Scholar]
- 7.Nauseef WM. Nox enzymes in immune cells. Semin Immunopathol. 2008 doi: 10.1007/s00281-008-0117-4. [DOI] [PubMed] [Google Scholar]
- 8.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 10.Jurkowska M, Bernatowska E, Bal J. Genetic and biochemical background of chronic granulomatous disease. Arch Immunol Ther Exp (Warsz) 2004;52:113–120. [PubMed] [Google Scholar]
- 11.Dinauer MC, Pierce EA, Bruns GA, Curnutte JT, Orkin SH. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990;86:1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 13.de Boer M, Singh V, Dekker J, Di Rocco M, Goldblatt D, Roos D. Prenatal diagnosis in two families with autosomal, p47(phox)-deficient chronic granulomatous disease due to a novel point mutation in NCF1. Prenat Diagn. 2002;22:235–240. doi: 10.1002/pd.296. [DOI] [PubMed] [Google Scholar]
- 14.Borgato L, Bonizzato A, Lunardi C, Dusi S, Andrioli G, Scarperi A, Corrocher R. A 1.1-kb duplication in the p67-phox gene causes chronic granulomatous disease. Hum Genet. 2001;108:504–510. doi: 10.1007/s004390100526. [DOI] [PubMed] [Google Scholar]
- 15.Aoshima M, Nunoi H, Shimazu M, Shimizu S, Tatsuzawa O, Kenney RT, Kanegasaki S. Two-exon skipping due to a point mutation in p67-phox--deficient chronic granulomatous disease. Blood. 1996;88:1841–1845. [PubMed] [Google Scholar]
- 16.Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, Levine JE, Petryniak B, Derrow CW, Harris C, Jia B, Zheng Y, Ambruso DR, Lowe JB, Atkinson SJ, Dinauer MC, Boxer L. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. [PubMed] [Google Scholar]
- 17.Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, Gonzalez-Aller C, Hiester A, deBoer M, Harbeck RJ, Oyer R, Johnson GL, Roos D. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci U S A. 2000;97:4654–4659. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen-Wolff A, Soldan W, Heyne K, Bickhardt J, Gahr M, Roesler J. Increased susceptibility of a carrier of X-linked chronic granulomatous disease (CGD) to Aspergillus fumigatus infection associated with age-related skewing of lyonization. Ann Hematol. 2001;80:113–115. doi: 10.1007/s002770000230. [DOI] [PubMed] [Google Scholar]
- 19.Moellering RC, Jr, Weinberg AN. Persistent Salmonella infection in a female carrier for chronic granulomatous disease. Ann Intern Med. 1970;73:595–601. doi: 10.7326/0003-4819-73-4-595. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki S, Shin H, Goya N, Nakagawara A. Identification of a carrier mother of a female patient with chronic granulomatous disease. J Pediatr. 1976;89:784–786. doi: 10.1016/s0022-3476(76)80807-4. [DOI] [PubMed] [Google Scholar]
- 21.Mills EL, Rholl KS, Quie PG. X-linked inheritance in females with chronic granulomatous disease. J Clin Invest. 1980;66:332–340. doi: 10.1172/JCI109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lun A, Roesler J, Renz H. Unusual late onset of X-linked chronic granulomatous disease in an adult woman after unsuspicious childhood. Clin Chem. 2002;48:780–781. [PubMed] [Google Scholar]
- 23.Dusi S, Poli G, Berton G, Catalano P, Fornasa CV, Peserico A. Chronic granulomatous disease in an adult female with granulomatous cheilitis. Evidence for an X-linked pattern of inheritance with extreme lyonization. Acta Haematol. 1990;84:49–56. doi: 10.1159/000205028. [DOI] [PubMed] [Google Scholar]
- 24.Curnutte JT, Hopkins PJ, Kuhl W, Beutler E. Studying X inactivation. Lancet. 1992;339:749. doi: 10.1016/0140-6736(92)90653-k. [DOI] [PubMed] [Google Scholar]
- 25.Anderson-Cohen M, Holland SM, Kuhns DB, Fleisher TA, Ding L, Brenner S, Malech HL, Roesler J. Severe phenotype of chronic granulomatous disease presenting in a female with a de novo mutation in gp91-phox and a non familial, extremely skewed X chromosome inactivation. Clin Immunol. 2003;109:308–317. doi: 10.1016/j.clim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Lyon MF. Sex chromatin and gene action in the mammalian X-chromosome. Am J Hum Genet. 1962;14:135–148. [PMC free article] [PubMed] [Google Scholar]
- 27.Puck JM, Willard HF. X inactivation in females with X-linked disease. N Engl J Med. 1998;338:325–328. doi: 10.1056/NEJM199801293380611. [DOI] [PubMed] [Google Scholar]
- 28.Salstrom JL. X-inactivation and the dynamic maintenance of gene silencing. Mol Genet Metab. 2007;92:56–62. doi: 10.1016/j.ymgme.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Burritt JB, Quinn MT, Jutila MA, Bond CW, Jesaitis AJ. Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J Biol Chem. 1995;270:16974–16980. doi: 10.1074/jbc.270.28.16974. [DOI] [PubMed] [Google Scholar]
- 30.Leto TL, Garrett MC, Fujii H, Nunoi H. Characterization of neutrophil NADPH oxidase factors p47-phox and p67-phox from recombinant baculoviruses. J Biol Chem. 1991;266:19812–19818. [PubMed] [Google Scholar]
- 31.Nakamura M, Murakami M, Koga T, Tanaka Y, Minakami S. Monoclonal antibody 7D5 raised to cytochrome b558 of human neutrophils: immunocytochemical detection of the antigen in peripheral phagocytes of normal subjects, patients with chronic granulomatous disease, and their carrier mothers. Blood. 1987;69:1404–1408. [PubMed] [Google Scholar]
- 32.Wang D, Pabst KM, Aida Y, Pabst MJ. Lipopolysaccharide-inactivating activity of neutrophils is due to lactoferrin. J Leukoc Biol. 1995;57:865–874. doi: 10.1002/jlb.57.6.865. [DOI] [PubMed] [Google Scholar]
- 33.Sergeant S, Waite KA, Heravi J, McPhail LC. Phosphatidic acid regulates tyrosine phosphorylating activity in human neutrophils: enhancement of Fgr activity. J Biol Chem. 2001;276:4737–4746. doi: 10.1074/jbc.M006571200. [DOI] [PubMed] [Google Scholar]
- 34.Cassidy LF, Lyles DS, Abramson JS. Depression of polymorphonuclear leukocyte functions by purified influenza virus hemagglutinin and sialic acid-binding lectins. J Immunol. 1989;142:4401–4406. [PubMed] [Google Scholar]
- 35.Sergeant S, McPhail LC. Opsonized zymosan stimulates the redistribution of protein kinase C isoforms in human neutrophils. J.Immunol. 1997;159:2877–2885. [PubMed] [Google Scholar]
- 36.Caldwell SE, McCall CE, Hendricks CL, Leone PA, Bass DA, McPhail LC. Coregulation of NADPH oxidase activation and phosphorylation of a 48-kD protein(s) by a cytosolic factor defective in autosomal recessive chronic granulomatous disease. J.Clin.Invest. 1988;81:1485–1496. doi: 10.1172/JCI113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 38.Qualliotine-Mann D, Agwu DE, Ellenburg MD, McCall CE, McPhail LC. Phosphatidic acid and diacylglycerol synergize in a cell-free system for activation of NADPH oxidase from human neutrophils. J Biol Chem. 1993;268:23843–23849. [PubMed] [Google Scholar]
- 39.Massey V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochimica et Biophysica Acta. 1959;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- 40.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 41.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 42.Emmendorffer A, Nakamura M, Rothe G, Spiekermann K, Lohmann-Matthes ML, Roesler J. Evaluation of flow cytometric methods for diagnosis of chronic granulomatous disease variants under routine laboratory conditions. Cytometry. 1994;18:147–155. doi: 10.1002/cyto.990180306. [DOI] [PubMed] [Google Scholar]
- 43.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- 44.McPhail LC, Harvath L, Abramson JS, Wheeler JG. The Neutrophil. Oxford: Oxford University Press; 1993. Signal transduction in neutrophil oxidative metabolism and chemotaxis; pp. 63–107. [Google Scholar]
- 45.Curnutte JT. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985;75:1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roesler J, Hecht M, Freihorst J, Lohmann-Matthes ML, Emmendorffer A. Diagnosis of chronic granulomatous disease and of its mode of inheritance by dihydrorhodamine 123 and flow microcytofluorometry. Eur J Pediatr. 1991;150:161–165. doi: 10.1007/BF01963557. [DOI] [PubMed] [Google Scholar]
- 47.Crockard AD, Thompson JM, Boyd NA, Haughton DJ, McCluskey DR, Turner CP. Diagnosis and carrier detection of chronic granulomatous disease in five families by flow cytometry. Int Arch Allergy Immunol. 1997;114:144–152. doi: 10.1159/000237660. [DOI] [PubMed] [Google Scholar]
- 48.Amos-Landgraf JM, Cottle A, Plenge RM, Friez M, Schwartz CE, Longshore J, Willard HF. X chromosome-inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet. 2006;79:493–499. doi: 10.1086/507565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 50.Parkos CA, Dinauer MC, Walker LE, Allen RA, Jesaitis AJ, Orkin SH. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A. 1988;85:3319–3323. doi: 10.1073/pnas.85.10.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chollet-Martin S, Lopez A, Gaud C, Henry D, Stos B, El Benna J, Chedevile G, Gendrel D, Gougerot-Pocidalo MA, Grandchamp B, Gerard B. Severe X-linked chronic granulomatous disease in two unrelated females. Eur J Pediatr. 2007;166:153–159. doi: 10.1007/s00431-006-0211-3. [DOI] [PubMed] [Google Scholar]
- 52.Francke U. Random X inactivation resulting in mosaic nullisomy of region Xp21.1----p21.3 associated with heterozygosity for ornithine transcarbamylase deficiency and for chronic granulomatous disease. Cytogenet Cell Genet. 1984;38:298–307. doi: 10.1159/000132078. [DOI] [PubMed] [Google Scholar]
- 53.Koker MY, Sanal O, de Boer M, Tezcan I, Metin A, Tan C, Ersoy F, Roos D. Skewing of X-chromosome inactivation in three generations of carriers with X-linked chronic granulomatous disease within one family. Eur J Clin Invest. 2006;36:257–264. doi: 10.1111/j.1365-2362.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- 54.Wolach B, Scharf Y, Gavrieli R, de Boer M, Roos D. Unusual late presentation of X-linked chronic granulomatous disease in an adult female with a somatic mosaic for a novel mutation in CYBB. Blood. 2005;105:61–66. doi: 10.1182/blood-2004-02-0675. [DOI] [PubMed] [Google Scholar]
- 55.Dean W, Lucifero D, Santos F. DNA methylation in mammalian development and disease. Birth Defects Res C Embryo Today. 2005;75:98–111. doi: 10.1002/bdrc.20037. [DOI] [PubMed] [Google Scholar]
- 56.Heyworth PG, Curnutte JT, Rae J, Noack D, Roos D, van Koppen E, Cross AR. Hematologically important mutations: X-linked chronic granulomatous disease (second update) Blood Cells Mol Dis. 2001;27:16–26. doi: 10.1006/bcmd.2000.0347. [DOI] [PubMed] [Google Scholar]