Abstract
Anti-inflammation immunotherapy has been successfully applied for the treatment of autoimmune diseases. Mucosal vaccines against autoimmune disorders are beneficial by influencing the regulatory compartment of gut and systemic adaptive immune systems. A Salmonella vector expressing colonization factor antigen I (CFA/I), shown to behave as an anti-inflammatory vaccine, stimulates the production of CD4+ CD25+ T cells and regulatory cytokines. In this work, we queried whether Salmonella-CFA/I can protect DBA/1 mice from collagen-induced arthritis (CIA). The incidence of arthritis and cartilage loss in vaccinated DBA/1 mice was remarkably lower when compared to unprotected mice. Clinical findings were accompanied by the suppression of inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-27. Vaccination evoked a multi-tier response consisting of IL-4 producing Th2 cells, an increased production of TGF-β by CD4+ T cells, and suppression of collagen II (CII)-specific CD4+ T cell proliferation. To assess the contribution of Salmonella-CFA/I-primed CD4+ T cells, adoptive transfer studies with total CD4+, CD4+CD25−, or CD4+ CD25+ T cells were performed 15 days post-challenge. Mice receiving either subset showed reduced disease incidence and low clinical scores; however, mice receiving total CD4+ T cells showed delayed disease onset by 10 days with reduced clinical scores, reduced IL-17 and IL-27, but enhanced IL-4, IL-10, IL-13, and TGF-β. Inhibition of TGF-β or IL-4 compromised protective immunity. These data show that Salmonella-CFA/I vaccination of DBA/1 mice protects against CIA by stimulating TGF-β- and IL-4-producing regulatory CD4+ T cells.
Keywords: Th1/Th2 cells, tolerance, vaccination, mucosa
Introduction
Targeted immunotherapy is a highly developed approach for treatment of chronic infections, autoimmune diseases, allograft rejections, and malignancies (1–3). Immunotherapy for autoimmune disorders is also especially attractive for correcting inflammatory diseases without having to resort to immunosuppressive drug therapies. The two main goals of such an approach are 1) to “switch off” the immune response against the host’s own tissues and 2) to maximally balance the relationships between effector cells of different lineages to prevent relapses of chronic inflammation.
Rheumatoid arthritis (RA)3 is a systemic inflammatory disease of the joints that disables almost half of the affected patients. The etiology of RA is still unknown, but hereditary factors and possible infectious agents (bacteria and viruses) are assumed to participate in the disease initiation (4). RA is mediated by T cells, predominantly CD4+ T cells, and proinflammatory cytokines, such as TNF-α and IL-1, are considered responsible for orchestrating pathogenesis (5–7). Using anti-TNF-α antagonists has resulted in success when combined with cytostatic therapy (8). The design of vaccines capable of preventing or reversing chronic inflammation is of particular interest. Collagen-induced arthritis (CIA), a model of RA, can be induced upon immunization with heterologous collagen II (CII) in DBA/1 mice or by mAbs to CII combined with LPS (9–11). CIA shares with RA several critical characteristics of the disease pathogenesis, including CD4+ T cells’ mediated inflammation and extensive cartilage and bone damage, resulting in joint deformities. This similarity permits the use of the CIA model as an investigative tool to test novel approaches for prevention and treatment of RA.
Live attenuated, Salmonella vaccine vectors are widely used as delivery systems and demonstrate avirulence, safety, and the ability to induce effective immune responses not only to Salmonella but also to passenger Ags (12). The Salmonella vector delivers the Ag directly to innate immune cells, thus, enhancing the development of the adaptive immune responses. Along these lines, oral immunization with our diarrheal vaccine for enterotoxigenic E. coli (ETEC), Salmonella expressing colonization factor Ag I, (Salmonella-CFA/I), induces strong mucosal and serum Ab responses against CFA/I fimbriae (13,14). This immunity is supported by an induction of a sustained Th2 cell activation against CFA/I fimbriae (14), much like that against soluble Ags, followed by Th1 (IFN-γ) cytokine production. Such a biphasic response induced by Salmonella-CFA/I is atypical because intracellular infections with Salmonella generally induce Th1-type responses (15). To ascertain whether this delay in Th1 cell development was somehow related to detective infection of innate cells, subsequent studies evaluated the vaccine’s ability to infect macrophages and showed an absence of proinflammatory cytokine production despite similar bacterial loads in macrophages (16). The presence of CFA/1 fimbriae on the Salmonella vector’s cell surface does not interfere in stimulating protective immunity to wild-type Salmonella, since the fimbriated Salmonella still provided similar survival efficacy as did Salmonella vector-immunized mice (17). Thus, Salmonella-CFA/I vaccine has the unique properties to protect against both wild-type Salmonella and ETEC.
Given these findings, we hypothesized that Salmonella-CFA/I is an anti-inflammatory vaccine in addition to having vaccine qualities for diarrheal diseases. When the anti-inflammatory properties of Salmonella-CFA/I were tested in an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), Salmonella-CFA/I-vaccinated SJL mice were protected against proteolipid protein (PLP139–151) challenge (18). This protection was mediated via Th2-type cytokines IL-4 and IL-13, as well as by TGF-β-producing regulatory CD4+CD25+ T (Treg) cells and a reduction in IL-17 (18, 19).
In this present work, we questioned whether oral immunization with Salmonella-CFA/I could inhibit the development of a different autoimmune disease, CIA, in DBA/1 mice. Such a study would address whether Salmonella-CFA/I could suppress the development of an autoimmune disease unrelated to myelin Ags and independent of auto-Ag in mice with a different genetic background. Oral immunization with Salmonella-CFA/I lowered CIA disease incidence and clinical scores. Salmonella-CFA/I-derived CD4+ T cells from both CD25− and CD25+ T cell subsets produced the regulatory cytokines IL-4, IL-10, and TGF-β that contributed to the observed protection, and this protection emanated optimally from unseparated CD4+ T cells. The protective effect by Salmonella-CFA/I was lost upon anti-TGF-β mAb treatment and significantly weakened by anti-IL-4 mAb treatment, indicating the importance of their role to suppress autoimmune disease. Thus, these results show that Salmonella-CFA/I can modify host immunity to auto-Ag independently of the auto-Ag.
Materials and Methods
Mice
DBA/1 male 6–8 weeks old mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were maintained at Montana State University Animal Resources Center in individual ventilated cages under HEPA-filtered barrier conditions and were fed sterile food and water ad libitum. All animal care and procedures were in accordance with institutional policies for animal health and well-being.
Immunizations and clinical evaluations of CIA
The Δasd Salmonella enterica serovar Typhimurium-CFA/I vaccine (strain H696) and its isogenic Salmonella vaccine vector (strain H647) were used for these studies (13, 14). Mice were vaccinated per os with 5x109 CFU Salmonella-CFA/I or its isogenic strain H647 (without CFA/I operon) seven days prior to CII challenge. Control mice received sterile PBS. For induction of arthritis, 100 μg of bovine CII (Chondrex, Inc., Redmond, WA) emulsified in CFA containing 4 mg/ml of M. tuberculosis (Chondrex, Inc.) were injected subcutaneously in the tail 2 cm from mouse body (20). Generally, by the end of week 5 post-challenge, 80–100% of mice in control group showed fully developed disease.
Mice were scored for clinical disease three times a week using a graded scale 0–3 for each limb for a maximal total score of 12 possible, as previously described (21): 0 - normal; 1 - mild redness or swelling of single digits; 2 - significant swelling of ankle or wrist with erythema; 3 - severe swelling and erythema of multiple joints. Percent of animals with arthritic lesions in the group represent the incidence of arthritis. Average clinical score in the group reflects the severity of the disease.
Histopathological analysis
Fifty days after CII challenge, limbs were fixed in 10% neutral buffered formalin and decalcified in 5% formic acid for 3–6 days. The joints were embedded in paraffin and cut at 8 μm sections. Hematoxylin - eosin and toluidine blue staining were performed for each sample. Histopathological scores for each joint were determined on a graded scale 0 –3 similar to that previously described (22): 0 - no changes; 1 - synovial hyperplasia and mild inflammatory infiltration; 2 - pannus formation with cartilage degeneration; 3 - heavy inflammatory infiltration and debris in the joint, severe chondrocytes and cartilage matrix loss with new bone tissue substitution, bone destruction. Paws and knee joint scores were estimated, with a total maximum score of 18 possible per mouse. Cartilage degeneration was scored in toluidine blue-stained sections on a graded scale 0 – 3 similar to that previously described (23): 0 - no cartilage loss; 1 - minimal chondrocytes and proteoglycan loss in superficial zone; 2 - moderate chondrocytes and proteoglycan loss into middle zone but above tidemark; 3 - severe cartilage degeneration through tidemark.
Anti-CFA/1 and anti-CII Ab ELISA
Serum and fecal samples were collected on day 21 after immunization with Salmonella-CFA/1. Samples were tested against purified CFA/I fimbriae, as previously described (14, 17), and against CII (Chondrex, Inc.). Serum samples from mice with induced arthritis were collected on day 21 post-challenge with CII, and samples of dilute sera were added to microtiter wells (Maxisorp Immunoplate II microtiter plates; Nunc, Roskilde, Denmark) coated with 2 μg/ml of ELISA Grade bovine or mouse CII (Chondrex, Inc.). For both Ab ELISA assays, goat anti-mouse HRP-labeled IgG1, IgG2a, IgG2b, or IgA (Southern Biotechnology Associates, Inc., Birmingham, AL) were used as detecting Abs. Enzymatic reaction was developed with ABTS (Moss, Inc., Pasadena, CA). Optical density was read at 415 nm using ELx808 microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). Endpoint titer represents reciprocal logarithm of two for the serum dilution with OD equal or more 0.1 above negative control.
Cytokine ELISA
Upon termination of the study (day 50 post-induction of arthritis challenge), axillary, inguinal, popliteal lymph node (LN) cells were purified and total mononuclear cells (5x106 cells/ml) were cultured alone or with 50 μg/ml CII for 3 days in a complete medium: RPMI-1640 medium supplemented with 10% of FBS (Invitrogen, Corp., Carlsbad, CA), 2 mM L-glutamine, 50 μM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. TNF-α, IL-1β, IL-6, IL-17, and IL-27 were measured from culture supernatants (16). Briefly, wells were coated with purified anti-mouse mAbs: TNF-α (5 μg/ml; BD Pharmingen), anti-IL-1β (3 μg/ml; R&D Systems, Minneapolis, MN), anti-IL-6 (2 μg/ml; BD Pharmingen), anti-IL-17 (2 μg/ml; BD Pharmingen), and anti-IL-27p28 (5 μg/ml; R&D Systems). Following blocking with PBS containing 1% BSA for 2 hrs at 370C, supernatants were incubated overnight at 40C. For detection, 0.5 μg/ml of biotinylated anti-mouse TNF-α, IL-6, IL-17 (BD Pharmingen), or IL-1β, IL-27p28 (R&D Systems) were added to wells for 1.5 hr at 370C. Reactions were developed using 1:1000 HRP-goat anti-biotin Ab (Vector Laboratories, Inc., Burlingame, CA) for 1 hr at RT followed by ABTS substrate (Moss, Inc.) Recombinant mouse TNF-α, IL-6, IL-17, IL-27 (R&D Systems), and IL-1β (PeproTech, Rocky Hill, NJ) were used to generate standard curves.
T cell assays
Axillary, popliteal, and inguinal LNs were isolated on day 15 post-challenge. CD4+ T cells were purified using Dynal® Mouse CD4 Negative Isolation Kit (Invitrogen Corp, Carlsbad, CA) to > 95% purity. Purified CD4+ T cells were resuspended in complete medium, and 2.5 x 105 cells/well in 200 μl were restimulated with varying doses of T Cell Proliferation Grade CII (Chondrex, Inc.) in the presence of syngenic mitomycin C-treated antigen-presenting cells. After 48 h, cells were pulsed with 0.5 μCi/well of 3H-TdR for 18 h. Samples were harvested, and incorporated 3H-TdR was measured by scintillation counting.
To measure cytokine-forming cells (CFCs), CD4+ T cells were restimulated at 106/ml with 50 μg/ml CII for 3 days, then added to nitrocellulose-based wells (MultiScreen-HA; Millipore, Bedford, MA) coated with purified anti-mouse mAbs to IFN-γ, IL-4, IL-10, IL-17 (BD Pharmingen), IL-13, and TGF-β (R & D Systems), as previously described (18,19). After overnight incubation at 37° C, cells were removed, and to the washed wells, biotinylated anti-mouse IFN-γ, IL-4, IL-10, IL-17 mAbs (BD Pharmingen), and biotinylated TGF-β or IL-13 Abs (R&D Systems) were added. Reactions were developed after incubation with HRP-conjugated anti-biotin (Vector Laboratories, Inc.) with precipitable substrate, AEC (Moss, Inc.). Spots were enumerated and normalized per 106 cells.
To characterize the cytokine profiles of CD4+ T cells induced by Salmonella-CFA/I or those induced by Salmonella vector, on day 15 post-immunization, mesenteric LN (MLN) CD4+ T cells were sorted for CD4+CD25− and CD4+CD25+ T lymphocytes using CELLection™ Biotin Binder Kit (Invitrogen Corp.). Each CD4+ T cell subset (106 cells) was stimulated with 10 μg/ml of plate-bound anti-CD3 mAb (BD Pharmingen) and 10 μg/ml of soluble anti-CD28 mAb (BD Pharmingen). After culturing for 4 days at 37° C, supernatants were collected and analyzed for cytokine production. Cytokine-specific capture ELISAs were performed, as previously described (18, 19).
Adoptive transfer of CD4+, CD4+CD25−, or CD4+CD25+ T cells from Salmonella-vaccinated mice
Mice were immunized with Salmonella-CFA/I or Salmonella vector, as described above, on day 0. At the same time, CIA was induced in a separate group of mice. On day 15 post-immunization, LNs were harvested from Salmonella-CFA/I- or Salmonella vector-vaccinated mice, and CD4+ T cells, CD4+CD25−, and CD4+CD25+ T cells were further sorted using magnetic beads as described. Purity > 92% was achieved for all procedures. Mice induced with CIA 15 days earlier were injected i.v. with 2x106 CD4+ T cells or an equivalent amount of CD4+ T cell subset: ~1.5x106 CD4+CD25− T cells and ~5x105 CD4+CD25+ T cells.
In vivo treatment with anti-IL-4 and anti-TGF-β mAbs
Hybridomas producing the anti-mouse IL-4 mAb (clone 11B11; ATCC Manassas, VA) and anti-mouse TGF-β (clone 1D11.16.8; ATCC) were expanded in serum-free supplemented HB101 media (Irvine Scientific, Santa Ana, CA). The mAbs were purified from culture supernatants using protein G (Sigma-Aldrich Co.) affinity chromatography. CD4+ T cells from mice immunized with Salmonella-CFA/I were sorted for adoptive transfer into mice challenged 15 days earlier with CII, as described above. Corresponding anti-IL-4, anti-TGF-β, or rat IgG in vivo Ab treatments was conducted weekly starting on the day of adoptive transfer for a total dose of 1 mg per mouse i.p.
Flow cytometry analysis
On day 14 after immunization with Salmonella-CFA/I or Salmonella vector, head and neck LN (HNLN) and MLN were purified. Immunofluorescent staining for surface CD4, CD25, CD39, GITR, and intracellular FoxP3 was performed using fluorochrome-labeled mAbs: CD4-FITC, CD4-PE-CY7, CD25-PerCp-CY5.5, GITR-PE (BD Pharmingen), FoxP3-PE, FoxP3-AlexaFluor® 647 (eBioscience, San Diego, CA) and polyclonal rabbit anti-CD39 (H-85) IgG (Santa Cruz Biotechnology, Santa Cruz, CA) paired with anti-rabbit IgG-FITC or IgG-PE (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). For proliferation assay on day 14 after immunization with Salmonella-CFA/I, CD4+ T cells from pooled HNLN and peripheral LN (effectors) were re-stimulated with 10 μg/ml of CFA/I during 24 hrs. CII specific CD4+ T cells (responders) were purified on day 15 after induction of arthritis, labeled by CFSE, as previously described (24), and co-cultured with CFA/I-specific CD4+ T cells in the presence of irradiated (3000 rad) APCs and 50 μg/ml CII. The ratio of responders: effectors: APCs was equal (1:1:1). Total cell density in cultures was 3x106/ml, and mAbs against IL-4, TGF-β, and IL-10 were added to the corresponding cultures at 50 μg/ml. After 5 days, cells were harvested, washed, and then stained for CD4, CD25, FoxP3, and GITR. Fluorescence was acquired on FACS Caliber, LSRII, or Canto (BD Biosciences, Mountain View, CA). CellQuest (BD Biosciences) and FlowJo (Tree Star, Inc., Ashland, OR) software was used for analysis.
Statistics
Fisher’s exact probability test was applied for incidence of arthritis. Mann-Whitney U-test was used for statistical evaluation of clinical scores, histology scores, and cartilage destruction. Student’s t-test was performed to analyze T cells proliferation, flow cytometry data, CFC responses, CII- and cytokine-specific ELISA measurements. Results were considered statistically significant if P-value was less than 0.05.
Results
Oral immunization with Salmonella-CFA/I inhibits development of CIA
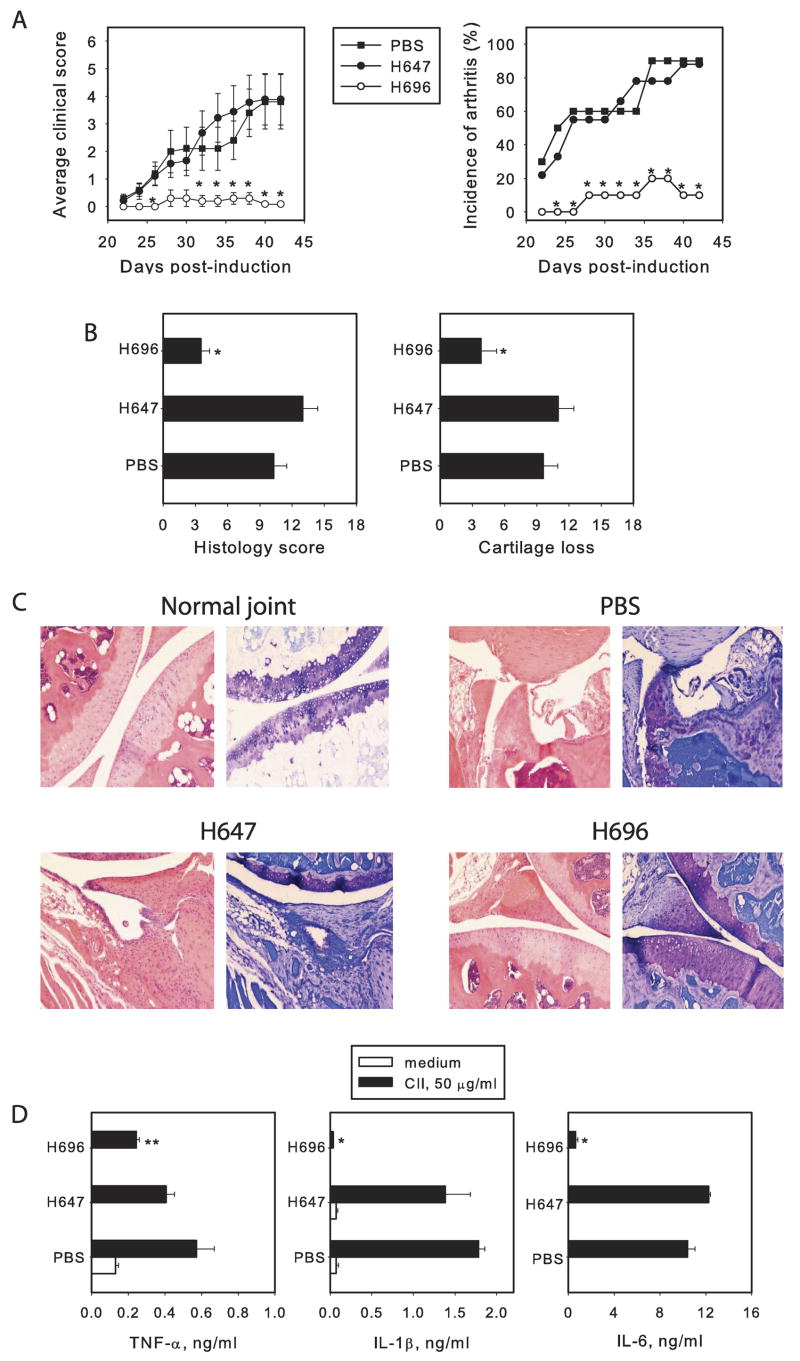
To test if Salmonella-CFA/I vaccine could result in protection against CIA, DBA/1 mice were orally vaccinated with 5x109 CFU of Salmonella-CFA/I (strain H696), and then CIA was induced one wk later. Control mice were given the isogenic Salmonella vector (strain H647) or sterile PBS. Three wks following CII challenge, ~ 90% of the PBS-dosed group developed CIA with a maximum average clinical score of ~ 4 (Fig. 1A). In the Salmonella-CFA/I-dosed group, a delay onset was observed of clinical symptoms with maximal incidence of arthritis being only ~ 20%, a remarkable suppression of CIA.
FIGURE 1.
Oral immunization with Salmonella-CFA/I (H696) suppresses the development of CIA. Eight-week-old DBA/1 male mice were orally vaccinated with Salmonella-CFA/I (H696), Salmonella vaccine vector (H647), or sterile PBS. (A) average clinical score and incidence of arthritis in mice challenged with bovine CII 7 days after oral vaccination. Data depict the mean of 10 mice per group ± SEM. Fisher’s exact probability test was applied to analyze the incidence of arthritis; Mann-Whitney U-test was performed for clinical score analysis; *P <0.05. (B) Histological scores determined on H-E stained sections and level of cartilage loss evaluated on sections stained with toluidine blue in protected and unprotected mice. Results depict the mean ± SEM, and statistical significance of P < 0.05 was obtained using a Mann-Whitney U-test. (C) Knees and paws joints sections were prepared on day 50 after challenge with bovine CII and stained with hematoxylin-eosin (left) and toluidine blue (right). The images are representative for each group from three experiments. Normal knee sections with undamaged cartilage; erosive bones and severe cartilage degeneration in PBS control group; arthritis developed in mice immunized with Salmonella vector; synovial hyperplasia and mild edema in the knee joint of mice vaccinated with Salmonella-CFA/I (H696). (D) Immunization with Salmonella-CFA/I results in suppression of proinflammatory cytokines TNF-α, IL-1β, and IL-6. Mice were immunized and challenged with CII as described. Total LN mononuclear cells were purified on day 50 post-challenge and stimulated for 3 days with CII. Cytokines concentrations were determined from collected supernatants by ELISA. * p< 0.001 as compared to PBS group and mice immunized with Salmonella vector; ** p< 0.05 as compared to PBS group and mice immunized with Salmonella vector. Student’s t-test was used for statistical analysis. One of 3 experiments is depicted.
To determine whether oral immunization with Salmonella-CFA/I reduces joint degeneration, on day 50 after challenge, paw and knee joint sections were stained with H & E to evaluate the histopathology or stained with toluidine blue to assess the degree of cartilage degeneration, characterized by chondrocytes and proteoglycan matrix loss. In comparison with normal joint sections, Salmonella-CFA/I-immunized mice developed edema and synovial hyperplasia (Fig. 1B and C), in contrast to median samples from PBS-dosed mice that showed severe bone and cartilage erosions and deformities (Fig. 1B and C). Consistent with clinical observations, both the average histological score and the level of cartilage degeneration were substantially lower in the group immunized with Salmonella-CFA/I, as compared with both control groups (Fig. 1B).
To compare production of proinflammatory cytokines between Salmonella-CFA/I-immunized mice and control groups, draining axillary, popliteal, and inguinal LNs were collected 50 days post-challenge, and total mononuclear cells were restimulated with CII. Confirming the clinical and histological findings, the results showed significant suppression of TNF-α, IL-1β, and IL-6 production by mononuclear cells from mice vaccinated with Salmonella-CFA/I compared to mice vaccinated with Salmonella vector or dosed with PBS. Thus, oral Salmonella-CFA/I immunization can prevent the development of CIA.
Salmonella-CFA/I does not influence anti-CII Ab responses
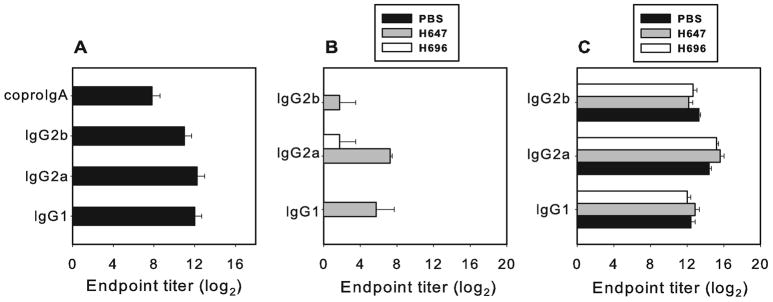
Oral immunization with Salmonella-CFA/I produces the expected anti-CFA/I Ab titers in DBA/1 mice (Fig. 2A), similar to that obtained with other mouse strains (13, 14, 17, 18). Likewise, oral immunization with this vaccine does not induce production of Abs which cross-react to CII (Fig. 2B).
FIGURE 2.
Salmonella-CFA/1 induces Ab response to (A) CFA/I without cross-reaction with (B) CII. Sera and fecal samples were collected on day 28 after oral immunization with 5x109 CFU of Salmonella-CFA/I and tested in ELISA against CFA/I or CII. Results represent the mean of 10 mice per group ± SEM. (C) Oral immunization with Salmonella-CFA/I or Salmonella vector does not change Ab response to CII. Sera samples were collected on day 21 after induction of arthritis. Results depict the mean of 10 mice per group ±SEM.
The cartilage destruction in arthritis joints occurs as a result of an inflammatory response for which the production of anti-CII Abs is required (25). To determine if immunization with Salmonella-CFA/I protects from excessive inflammation by altering the Ab response to CII, serum samples were collected on day 21 post-induction of arthritis, and Ab titers to CII were measured. All groups developed equivalent IgG1, IgG2a, and IgG2b responses to CII (Fig. 2C). These data demonstrate that immunization with Salmonella-CFA/I does not inhibit anti-CII Ab production.
Oral immunization with Salmonella-CFA/I reduces CII-specific CD4+ T cell proliferation and increases Th2-type/regulatory cytokine production
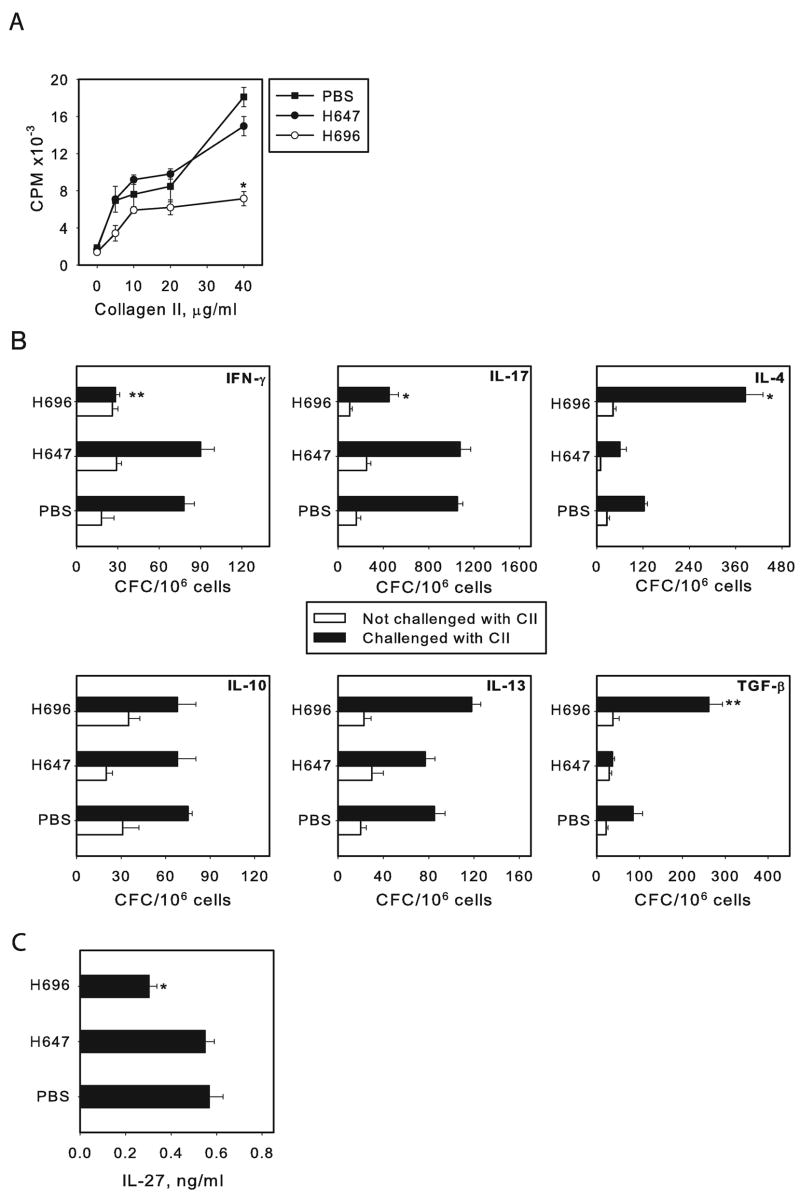
To assess the CD4+ T cell responses to CII, CD4+ T cells from diseased and protected mice were tested. Purified CD4+ T cells restimulated with CII showed a dose-dependent proliferative response in all groups, but in mice that were immunized with Salmonella-CFA/I, this response was significantly reduced, unlike that obtained with CD4+ T cells from mice given the Salmonella vector or PBS (Fig. 3A). To determine the types of cytokines produced upon CII restimulation, cytokine ELISPOT assays were performed to quantify IFN-γ, IL-4, IL-10, IL-13, IL-17, and TGF-β cytokine-forming cells (CFCs) one week before first symptoms of arthritis appeared (day 15 post-challenge). Purified LN CD4+ T cells restimulated in vitro with CII for 72 hr resulted in a 3- and 4-fold increase of CII-specific CD4+ T cells producing TGF-β and IL-4, respectively, by Salmonella-CFA/I-vaccinated mice when compared to PBS- and vector-immunized mice (P ≤ 0.01; Fig. 3B). No significant differences in IL-13 or IL-10 CFC were observed between Salmonella-CFA/I, H647-immunized groups and the PBS-dosed group. Significant suppression of IFN-γ- and IL-17-producing CD4+ T cells from Salmonella-CFA/I-vaccinated mice when compared to Salmonella-vector immunized mice or PBS group was also noted (Fig. 3B). Along with the decreased Th1 and Th17 cell activities, suppressed production of IL-27 was observed by whole LN cultures derived from protected mice upon in vitro CII restimulation (Fig. 3C). These results demonstrated that the Salmonella-CFA/I vaccine is capable of altering the function of CII- specific CD4+ T cells, driving the development of IL-4- and TGF-β-producing CD4+ T cells, accompanied by reduction of IFN-γ, IL-17, and IL-27 production.
FIGURE 3.
Immunization with Salmonella-CFA/I alters CII-specific CD4+ T cell responses. (A) Mice were immunized with Salmonella-CFA/I (H696), Salmonella vector (H647), or given sterile PBS. Challenge with CII was performed on day 7 post-immunization. Axillary, inguinal, and popliteal LN were collected 15 days after challenge, and purified CD4+ T cells were restimulated in culture with an equivalent amount of mitomycin C-treated syngenic APCs in the presence of varying amounts of CII for 72 h. Depressed CII-specific proliferative responses by draining LN CD4+ T cells were obtained upon restimulation with CII. Data represent the mean in CPM of five replicates ± SEM, and data are representative of three experiments. (B) Cytokine ELISPOT for draining LN CD4+ T cell responses upon CII restimulation. Open bars show cytokine producing cells from mice in which arthritis was not induced. Data depict the mean CFC ± SEM and are representative of three experiments. *P < 0.001 as compared to PBS control and Salmonella vector-immunized group; **P < 0.01 as compared to PBS control and Salmonella vector-immunized group. (C) Immunization with Salmonella-CFA/I results in suppressed IL-27 response to CII upon restimulation of draining LN cells in vitro. * P < 0.01 as compared to PBS and Salmonella vector groups.
Therapeutic potential of CD25+ or CD25− CD4+T cells from Salmonella-CFA/I-vaccinated mice against CIA
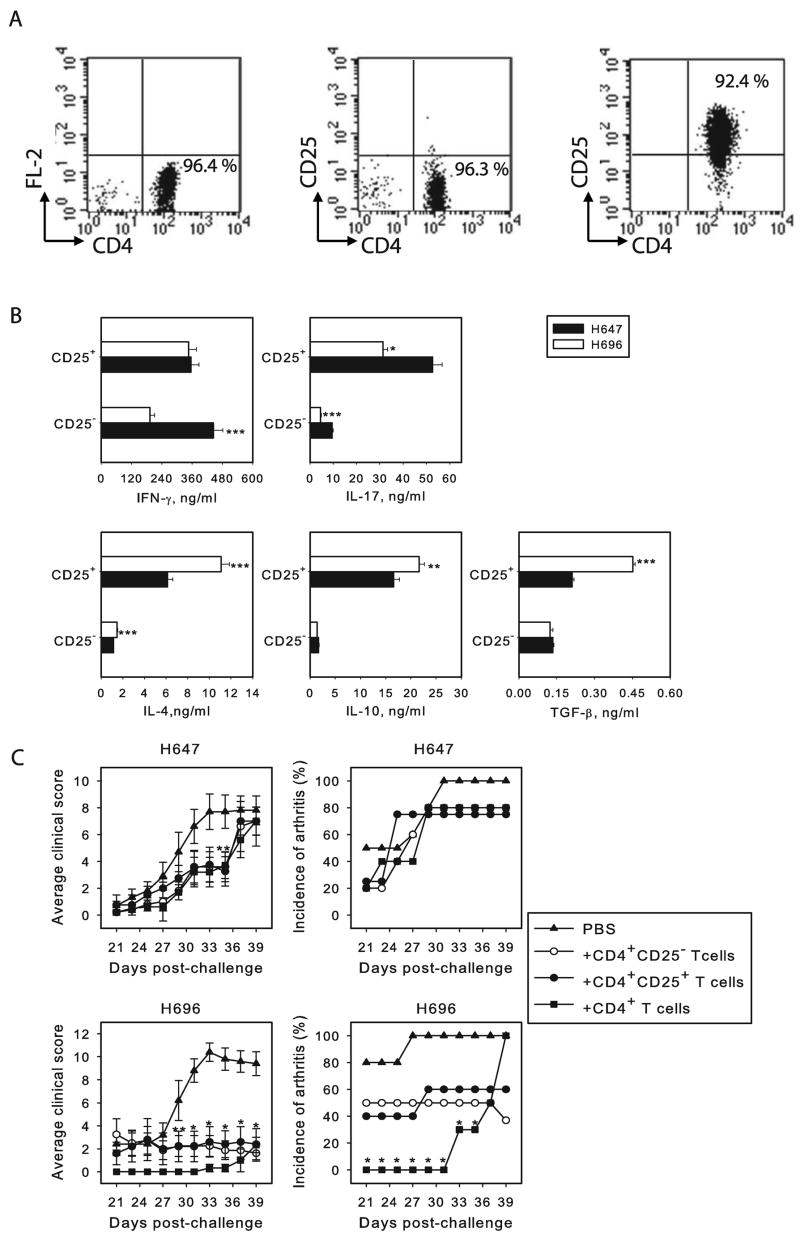
Since immunization with Salmonella-CFA/I results in Th2-type and regulatory cytokine responses to CII, we queried which CD4+ T cell subset from Salmonella-CFA/I-immunized mice is responsible for protecting against the development of CIA. To determine the activity of MLN CD4+CD25− and CD4+CD25+ T cells induced by Salmonella-CFA/I or Salmonella vector, these T cell subsets were purified on day 15 after immunization and stimulated in vitro with plate-bound anti-CD3 and soluble anti-CD28 mAbs to assess cytokine profiles. IFN-γ was equally produced by CD4+CD25+ T cells from Salmonella-CFA/I- and Salmonella vector-immunized mice, but its production by CD4+CD25− T cells was significantly (P<0.001) lower in Salmonella-CFA/I-vaccinated mice (Fig. 4B). IL-17 was significantly reduced (P < 0.05) in both CD4+ T cell subsets from Salmonella-CFA/I-vaccinated mice (Fig 4B). Unlike Salmonella vector-immunized mice, the CD4+CD25+ T cells from Salmonella-CFA/I-vaccinated mice showed elevated production of IL-4, IL-10, and TGF-β (Fig. 4B). While these anti-inflammatory cytokines were produced by CD4+CD25− T cells from both Salmonella-immunized groups, IL-4 was elevated (P < 0.001) by Salmonella-CFA/I-vaccinated mice (Fig. 4B), but IL-10 and TGF-β production were not significantly different. Thus, both CD4+ T cell subsets showed more of an anti-inflammatory response and increased FoxP3 expression in H696-vaccinated mice than those immunized with the Salmonella vector.
FIGURE 4.
CD4+ T cells activated by Salmonella-CFA/I are potent against CIA. (A) FACS profiles for sorted CD4+, CD4+CD25− and CD4+CD25+ T cells. (B) Cytokine production by MLN CD4+CD25− and CD4+CD25+ T cells after immunization with Salmonella-CFA/I or Salmonella vector. Purified 106 CD4+CD25− and CD4+CD25+ T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs for 3 days. Data show the mean concentration of cytokine ± SEM in series of 6 measurements. *P < 0.001, **P < 0.005, ***P < 0.01 depict significant differences between corresponding Salmonella-CFA/I-and Salmonella vector-primed cells. (C) CD4+ T cells induced by Salmonella-CFA/I, but not by Salmonella vector, confer protection from CIA. Recipient CII challenged DBA/1 mice were adoptively transferred with purified CD4+, CD4+CD25−, or CD4+CD25+ T cell subsets previously primed with Salmonella-CFA/I or Salmonella vector. Adoptive transfer was performed 15 days after induction of CIA (8 mice per group). Severity of arthritis symptoms is represented as average clinical score per group. Incidence of arthritis corresponds to the percentage of mice with arthritis in each group. *P < 0.01 as compared to control; **P < 0.05 as compared to control.
Given these findings, adoptive transfer studies were performed using purified CD4+CD25−, CD4+CD25+, or whole CD4+ T cells from mice immunized with Salmonella-CFA/I or Salmonella vector (Fig. 4C) into CII-challenged mice on day 15 post-CII challenge. Disease progression was monitored, and it was found that in all recipients given Salmonella-CFA/I-primed cells, the average clinical scores were significantly less when compared to untreated control mice (Fig. 4C). In fact, the mice adoptively transferred with the total CD4+ T cells showed the best protection as opposed to mice given the individual CD4+CD25− or CD4+CD25+ T cell subsets. Interestingly, both CD4+CD25− and CD4+CD25+ T cells demonstrated a similar potential to reverse the development of arthritis symptoms. Incidence of arthritis in these groups was reduced by half (Fig. 4C); however, only the whole CD4+ T cells were able to delay the disease onset in 100% of mice for 10 days, and only low clinical scores were observed by day 40 (Fig. 4C). Notably, neither of the CD4+ T cell subsets derived from immunization with Salmonella vector could prevent CIA development when adoptively transferred to CII-challenged mice (Fig. 4C). Thus, these experiments showed that CD4+ T cells induced by Salmonella-CFA/I are able to protect against CIA during the development phase (15 days post-challenge with CII). This protection is equally conferred by either CD4+CD25− or CD4+CD25+ T cells, but the best protection required the participation of both CD4+ T cell subsets.
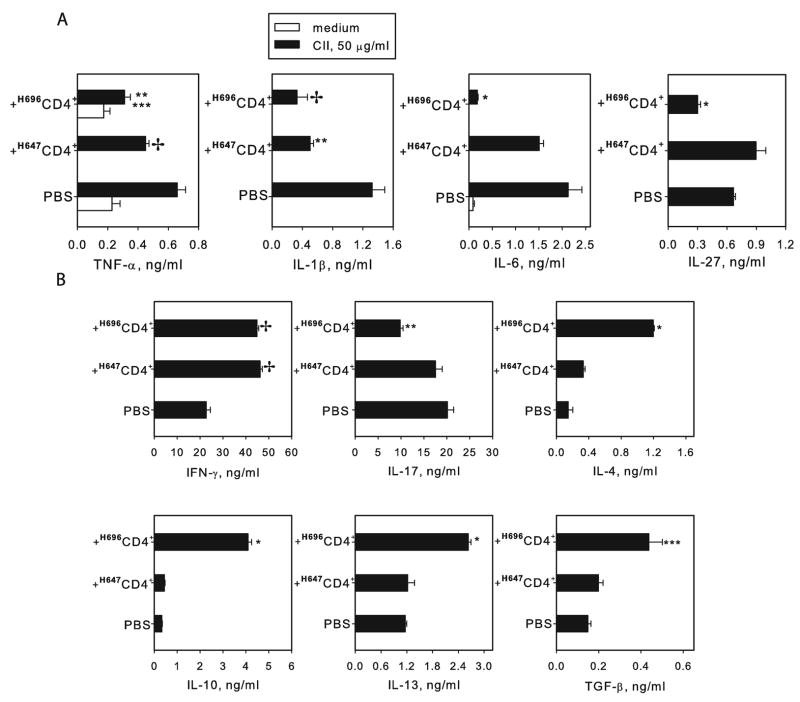
Upon termination of the study, draining LN mononuclear cells were isolated from recipients adoptively transferred with total CD4+ T cells from H696- or H647-primed mice, and were cultured in vitro without and with CII to assess proinflammatory cytokine production. TNF-α, IL-1β, IL-6, and IL-27 were significantly reduced in recipients given Salmonella-CFA/I-primed CD4+ T cells when compared to diseased (PBS-dosed) mice (Fig. 5A). Cytokine production, except for IL-27, by recipients given Salmonella vector-primed cells was also lower compared to diseased (PBS-dosed) mice, but TNF-α and especially IL-6 (which was still 5-fold greater) remained elevated when compared to recipients given Salmonella-CFA/I-primed CD4+ T cells (Fig. 5A).
FIGURE 5.
Suppression of proinflammatory cytokine production by draining LN cells after adoptive transfer of Salmonella-CFA/I-primed CD4+ T cells. (A) Total mononuclear cells from the three treatment groups at the termination of the study in Fig. 4 were purified and cultured for 3 days with CII. TNF-α, IL-1β, IL-6, and IL-27 levels were measured in supernatants by ELISA. Data represent the mean ±SEM of 6 replicates. * P < 0.001 as compared to PBS control and Salmonella vector (H647) primed CD4+ T cells recipients; ** P < 0.01, *** P < 0.05 as compared to PBS control; ✢ P < 0.05 as compared to Salmonella vector (H647) primed CD4+ T cells recipients. (B) Recipients of total CD4+ T cells from Salmonella-CFA/I-vaccinated mice show enhanced production of Th2-type and regulatory cytokines. CD4+ T cells purified from draining LN of recipients given either Salmonella-CFA/I-, Salmonella vector-primed CD4+ T cells, or PBS control mice (at the termination of the study in Fig. 4) were restimulated with CII in presence of syngenic APC for 5 days. Data represent mean cytokine concentration ± SEM from two experiments. * P <0.001, ** P <0.005, *** P <0.05 as compared to PBS group and recipients of Salmonella vector primed CD4+ T cells; ✢ P <0.001 as compared to PBS control.
In addition, CD4+ T cell cytokine production in recipient mice was also measured. Adoptive transfer of CD4+ T cells derived after Salmonella-CFA/I immunization caused an increase in production of Th2-type cytokines IL-4 and IL-13 and regulatory cytokines IL-10 and TGF-β by CD4+ T cells in recipient mice (Fig. 5B) with concomitant reduction in IL-17 (Fig 5B). Consistent with the observed clinical findings, Salmonella vector-primed CD4+ T cells could not alter the trend in enhanced Th2-type and regulatory cytokine production by recipients when compared to diseased (PBS-dosed) mice. Collectively, these experiments demonstrated the potential of Salmonella-CFA/I-derived CD4+ T cells to suppress CIA suggestively through Th2 cytokines, IL-10, and/or TGF-β.
Salmonella-induced CFA/I CD4+ T cells suppress CII-primed CD4+ T cells
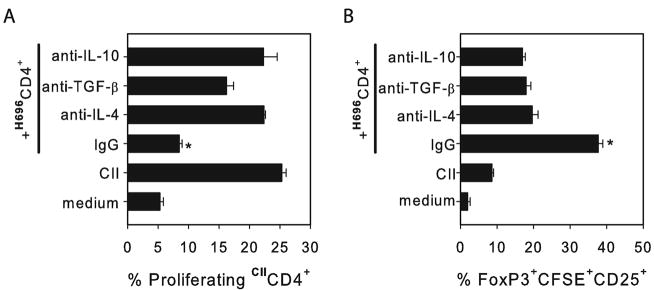
To evaluate the relative contribution of IL-4, IL-10, and TGF-β produced by Salmonella-CFA/I- induced CD4+ T cells in the suppression of autoimmune inflammation, an in vitro analysis was performed on CII-specific proliferation by effector CD4+ T cells. Salmonella-CFA/I CD4+ T cells were purified from combined LNs on day 14 post-immunization. These cells were restimulated with purified CFA/I fimbriae for 24 hrs in the presence of irradiated APC. CII primed-CD4+ T cells were purified on day 15 after arthritis challenge and labeled with CFSE. CFA/I-stimulated CD4+ T cells and CII-primed CFSE+ CD4+ cells were co-cultured in the presence of CII and irradiated APCs with inhibitory concentrations of anti-IL-4, anti-IL-10, or anti-TGF-β mAbs, or rat IgG. Flow cytometry analysis revealed that neutralization of each cytokine could restore proliferation of CFSE+CD4+ T cells when in the presence of inhibitory CFA/I-primed CD4+ T cells (Fig. 6A). This experiment demonstrates that IL-4, IL-10, and TGF-β can contribute to the observed suppression mediated by Salmonella-CFA/I-primed CD4+ T cells. Interestingly, the percentage of CFSE+ FoxP3+CD25+ CD4+ T cells induced by CFA/I-primed CD4+ T cells decreased nearly 2-fold if blocking mAbs were added to the co-cultures.
FIGURE 6.
The protective impact conferred by Salmonella-CFA/I-induced CD4+ T cells is abated upon anti-IL-4, anti-IL-10, or anti-TGF-β treatment resulting in the restoration of CII-specific CD4+ T cell proliferation. (A) Mice were immunized with Salmonella-CFA/I, as previously described, and 14 days later, MLN and HNLN CD4+ T cells were cell-sorted and restimulated with CFA/I fimbriae for 24 hrs. Effector CD4+ T cells from mice with CIA were purified 15 days after CII challenge and labeled with CFSE. These were added to CFA/I-restimulated CD4+ T cells in the presence of irradiated APCs, CII, and neutralizing quantities of anti-IL-4, anti-TGF-β, or anti-IL-10 mAbs or normal rat IgG. Flow cytometry analysis of CFSE+ CD4+ T cells was performed after 5 days of incubation. (B) Frequency of FoxP3 expression by CII-specific CFSE+ CD4+CD25+ T cells. Data represent the mean of three replicates from each culture ± SEM. Significant differences are depicted: *P<0.001 as compared to control and anti-IL-4, anti-TGF-β, or anti-IL-10 mAb-treated cells.
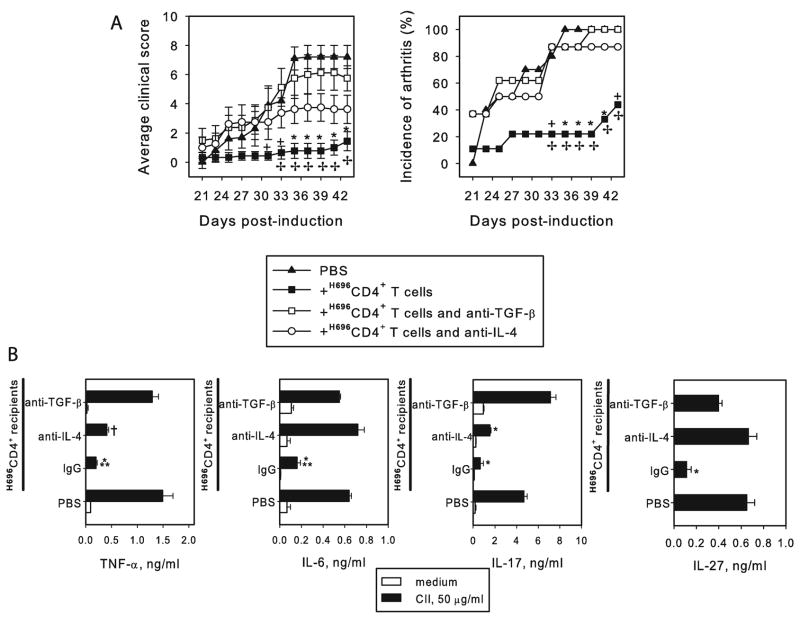
In vivo neutralization of IL-4 or TGF-β compromises the therapeutic capacity of Salmonella-CFA/I-primed CD4+ T cells against CIA
To further assess the role of IL-4 and TGF-β-producing CFA/I-primed CD4+ T cells in the protection against CIA, purified Salmonella-CFA/I-primed CD4+ T cells were adoptively transferred into recipients induced with CIA two wks earlier. Anti-IL-4 or anti-TGF-β mAb treatment was initiated at the time of adoptive transfer and treated subsequently at weekly intervals for a total of four doses. The in vivo neutralization of TGF-β resulted in clinical scores and incidences of CIA in these recipients similar to unprotected mice (Fig. 7A and Table I). Treatment with anti-IL-4 mAb resulted in significantly higher clinical scores and incidences of CIA to 90% (Fig. 7A). Although the clinical score of anti-IL-4 mAb-treated mice was lower than in PBS control mice or in anti-TGF-β-treated mice, the disease incidence was significant. Upon termination of the study, draining LN cells were purified from each test group and evaluated for proinflammatory cytokine production following CII restimulation. The amount of TNF-α, IL-6, IL-17, and IL-27 produced by anti-TGF-β mAb-treated mice was similar to the PBS control group (Fig. 7B). In the anti-IL-4 mAb-treated group, TNF-α and IL-17 were significantly greater than in recipient mice given rat IgG, and IL-6 and IL-27 production were similar to PBS control group (Fig.7B). Such an inflammatory cytokine profile in anti-IL-4 mAb-treated mice is also in consistent with clinical findings. Collectively, these studies show that in vivo anti-TGF-β mAb treatment at the time of adoptive transfer of protective Salmonella-CFA/I-derived CD4+ T cells (day 15 after induction of arthritis) completely reverses their anti-inflammatory effect. This demonstrates that TGF-β is essential for protection with the Salmonella-CFA/I-induced CD4+ T cells. Neutralization of IL-4 also compromises the protective properties of Salmonella-CFA/I, suggesting that IL-4 is also an important cytokine for protection against CIA.
FIGURE 7.
Treatment with anti-IL-4 or anti-TGF-β mAbs in vivo compromises protection to CIA by Salmonella-CFA/I-induced CD4+ T cells. (A) CD4+ T cells were purified from pooled HNLN and MLN of mice immunized with Salmonella-CFA/I 15 days post-immunization and adoptively transferred to mice in which CIA was induced 2 wks earlier. Corresponding Ab treatments were conducted at weekly intervals starting the day of adoptive transfer for a total of four doses. Each group contained 8 – 10 mice. * P < 0.005, + P < 0.05 as compared to PBS group; ✢ P < 0.05 as compared to anti-IL-4 or anti-TGF-β mAb-treated mice. (B) Restored production of proinflammatory cytokines in anti-IL-4 and anti-TGF-β mAb-treated mice. Draining LN were collected after following the disease course, and 5x106 cells/ml were re-stimulated with CII for 3 days. TNF-α, IL-6, and IL-17 were measured in supernatants by capture ELISA. Data depict mean value of 6 replicates ± SEM. * P < 0.005 as compared to PBS or anti-TGF-β mAb-treated group; ** P < 0.05 as compared to anti-IL-4 mAb-treated group; † P < 0.005 as compared to anti-TGF-β mAb-treated or PBS groups.
Table I.
Treatment with anti-TGF-β and anti-IL-4 mAbs weakens therapeutic potential of Salmonella-CFA/1 induced CD4+ T cells. a
| Treatment b | CIA/Total c | Day Onset d | Max. Score e | Average score f | CS g |
|---|---|---|---|---|---|
| PBS | 10/10 | 27.8 ± 1.6 | 10 | 7.2 ± 0.78 | 50.4 ± 7.11 |
| H696CD4+ T cells and IgG | 4/10 | 33 ± 5.35 | 4 | 1.44 ± 0.65* † | 7.78 ± 4.65* † |
| H696CD4+ T cells and anti-IL-4 | 8/8 | 26.71 ± 2.29 | 8 | 3.62 ± 0.96 | 34.87 ± 11.09 |
| H696CD4+ T cells and anti-TGF-β | 8/8 | 27.25 ± 2.43 | 12 | 5.75 ± 0.86 | 49.37 ± 10.13 |
Arthritis was induced in DBA/1 mice with 100 μg bovine CII in complete Freund’s adjuvant on day 0.
Salmonella-CFA/1 primed CD4+ T cells were adoptively transferred to mice with induced arthritis on day 15. Intra-peritoneal mAb treatments were initiated on day of transfer, then weekly for a total of four doses (1 mg total of mAb/mouse).
Number of mice with CIA /total in a group for 43 days after CII challenge.
Mean day ± SEM of first symptoms onset in diseased mice only.
Maximum score in group in entire observation period.
Average clinical score per group on day 43 post-induction (end of observation period) calculated as sum of individual scores divided by the number of mice in group ± SEM.
P < 0.005 as compared to PBS group;
P < 0.05 as compared to anti-IL-4 or anti-TGF-β mAb treated group.
Cumulative scores (CS) calculated as all scores during the period of observation divided by number of mice in each group.
P < 0.005 as compared to PBS and anti-TGF-β treated groups;
P < 0.05 as compared to anti-IL-4 treated group.
FoxP3 correlate with both CD25+ and CD25− CD4+ T cell subsets
Considering that Salmonella-CFA/I stimulates development of FoxP3+ CD4+CD25+ T cells responsible for protection from EAE (19), the CD4+ T cell phenotype was analyzed two wks after oral immunization with Salmonella-CFA/I or Salmonella vector vaccines. The frequency of CD4+CD25+ T cells in HNLN and MLN increased by 30% in mice immunized with Salmonella-CFA/I compared to PBS- and empty vector-immunized mice (Table II). Within this subset, the percentage of FoxP3 expressing cells was also higher (1.5-fold for HNLN and almost 2-fold for MLN). Since it was recently shown that CD39 could associate with FoxP3+ Treg cells (26, 27), CD39 expression was also evaluated on both Treg and CD4+ CD25−T cells. Nearly half of FoxP3+CD25+CD4+ T cells were also CD39 positive, whereas only about 10% of FoxP3−CD25+CD4+ T cells expressed CD39 after immunization with Salmonella-CFA/I. Additionally, expression of FoxP3 by MLN CD4+CD25− T cells induced by Salmonella-CFA/I was also higher (~2-fold) than in the corresponding subset from PBS and vector control mice, and about 1/3 of these FoxP3+ CD4+CD25− T cells was CD39 positive. Thus, we observed that the population of CD4+ T cells expressing FoxP3 included both the CD25+ and CD25−T cell subsets after vaccination with Salmonella-CFA/I.
Table II.
CD4+ T cells characterization on day 14 post-immunization with Salmonella-CFA/I
| CD4+ T Cell Subset | Head and Neck LN | MLN | ||||
|---|---|---|---|---|---|---|
| PBS | H647 | H696 | PBS | H647 | H696 | |
| %CD25+CD4+ | 9.60 ± 0.11 | 10.38 ± 0.29 | 12.87 ± 0.58† | 10.32 ± 0.23 | 9.37 ± 0.25 | 13.73 ±0.34* |
| %FoxP3+CD25+CD4+ | 9.96 ± 0.53 | 9.92 ± 1.19 | 14.96 ± 1.16† | 24.08 ± 4.82 | 21.93 ± 1.1 | 41.19 ± 2.97† |
| %CD39+CD25+CD4+ | 5.34 ± 0.76 | 6.38 ± 1.32 | 2.89 ± 1.38 | 4.96 ± 0.26 | 4.74 ± 1.40 | 16.53 ± 1.89‡ |
| %CD39+FoxP3+CD25+CD4+ | 14.21 ± 0.75 | 19.54 ± 2.79 | 17.54 ± 2.56 | 18.14 ± 2.06 | 13.70 ± 2.20 | 41.34 ± 2.44‡ |
| %CD39+FoxP3−CD25+CD4+ | 3.83 ± 0.42 | 4.82 ± 1.43 | 2.81 ± 0.14 | 6.64 ± 0.38 | 5.67 ± 1.44 | 12.5 ± 1.88† |
| %FoxP3+CD25−CD4+ | 1.30 ± 0.24 | 0.62 ± 0.08 | 1.88 ± 0.45 | 6.48 ± 0.63 | 4.89 ± 1.52 | 11.06 ± 2.5† |
| %CD39+CD25−CD4+ | 2.90 ± 0.75 | 3.42 ± 0.55 | 1.40 ± 0.07 | 3.05 ± 0.52 | 2.33 ± 0.77 | 15.03 ± 1.43* |
| %CD39+FoxP3+CD25−CD4+ | 9.75 ± 1.44 | 9.43 ± 1.65 | 8.41 ± 1.87 | 11.21 ± 0.95 | 8.77 ± 1.72 | 31.97 ± 2.67‡ |
| %CD39+FoxP3−CD25−CD4+ | 4.1 ± 1.12 | 3.51 ± 0.89 | 1.70 ± 0.23 | 4.42 ± 0.84 | 2.67 ± 0.74 | 17.44 ± 1.27* |
| %CD39+CD4+ | 1.92 ±3.16 | 3.81 ± 0.89 | 1.66 ± 0.22 | 3.02 ± 0.25 | 2.56 ± 0.61 | 16.59 ± 1.43* |
P < 0.001;
P < 0.005;
P < 0.05 as compared to PBS and H647 groups.
Discussion
Salmonella vaccine vectors have been successfully exploited for transporting vaccines into mucosal inductive sites, particularly for the GALT (12–15). Previously, we demonstrated that live Salmonella-based vaccines against ETEC can be protective not only from intestinal infection (28, 29), but at the same time have distinctive properties of soluble Ag delivery (14, 28, 29). These unique attributes include the absence of proinflammatory cytokine production following in vitro infection of macrophages (16), as well as induction of Th2-type cytokines, which provides an amplified in vivo secretory IgA response (14, 28, 29). Because of these anti-inflammatory properties, we questioned whether oral immunization with Salmonella-CFA/I vaccine could prevent CIA in susceptible DBA/1 mice.
CIA is an experimental inflammatory disease induced in susceptible mice challenged with heterologous CII in complete Freund’s adjuvant. CIA shares certain manifestations with RA, as evidenced by the production of proinflammatory cytokines (TNF-α, IL-1, and IFN-γ), Abs to CII, Th1 and other mononuclear cells infiltration, and irreversible joint degeneration (7, 30–32). Arthritis in mice can be passively transferred by CII-reactive mAbs in the presence of LPS or by collagen-reactive T cells (33, 34), proving the important role of both B and T cells in pathogenesis. This study was conducted to examine if the featured immune response to Salmonella-CFA/I could influence the development of specific immunity to CII and, consequently, inhibit systemic inflammation, such as CIA. Clearly from the results shown here, oral immunization of DBA/1 mice with Salmonella-CFA/I resulted in substantially reduced incidence of clinical CIA when compared to mice treated with PBS or with the isogenic Salmonella vector. Clinical observations were further supported by decreased production of proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-27 in draining LN cells of protected mice.
Microbial products can positively or negatively affect the clinical coarse of the joint pathology. Treatment of DBA/1 mice with E. coli heat-labile enterotoxin B subunit before challenge with CII was shown to prevent arthritis (35). Administration of E. coli or Salmonella LPS to mice with CIA leads to enhancement of clinical symptoms (36). Despite presence of LPS, the H696 strain was still protective, unlike its parental strain H647. Perhaps, the expression of CFA/I fimbriae interferes or minimizes the Salmonella’s LPS effect or, alternatively, interrupts the normal host recognition mechanisms specifically for LPS, as suggested in earlier studies (16).
Experiments with the EAE model showed that immunization with Salmonella vector could reduce demyelination (18, 19), although significantly less effective than the CFA/I-expressing (H696) strain. This partially protective effect rendered upon immunization with the Salmonella vector was not observed in the CIA model, and in fact, the average clinical score and disease frequency in this group was similar to PBS control.
One explanation that could account for reduced CIA in vaccinated mice is the suppressed production of anti-CII Abs, particularly IgG2a. However, published studies of others demonstrate that suppression of CIA does not always correlate with decreased anti-CII Ab responses (37). On the other hand, exacerbated arthritis in IL-10-deficient mice and IFN-γRμ-KO mice was observed despite lower anti-CII Abs (38, 39). Investigating B cell responses to CII, we found that Salmonella-CFA/I-vaccinated DBA/1 failed to show reduced anti-CII Ab titers in any of the IgG subclasses.
In contrast, protection conferred by Salmonella-CFA/I was mediated by suppressed proliferation of CII-specific CD4+ T cells and immune deviation in their response toward Th2-type and regulatory cytokine production. The cytokine profile for CII-restimulated CD4+ T cells confirmed that Salmonella-CFA/I redirects these immune responses to be Th2 cell-biased, as evidenced by increased numbers of IL-4 CFC. Similar immune deviation accounted for protecting SJL mice against PLP-mediated EAE (18, 19). However, in that model, IL-13 was substantially induced, more so than IL-4. In this current work, the amount of IL-13-producing CD4+ T cells by Salmonella-CFA/I-immunized mice was only slightly increased. This finding that IL-4 is protective against CIA is consistent with what others have reported to limit inflammation (40). In that previous study, DBA/1 mice that were adoptively transferred with tolerogenic splenocytes and treated with anti-IL-4 mAb lost their protection to CII challenge. Likewise, IL-4−/− mice could not be tolerized with CII, thus, emphasizing the relevance of IL-4 for protection against CIA.
The notion that regulatory cells could control CIA was alluded to in an early study by Kakimoto et al. in which they described a CII-specific T cell line that could suppress disease onset and disease severity when these cells were adoptively transferred into recipient DBA/1 mice (41). The adoptive transfer of those cells could, in a dose-dependent fashion, suppress CIA (41). More recently, in a similar vein, depletion of CD25+ T cells 2 wk prior to immunization with CII resulted in a more severe CIA in DBA/1 mice, and the adoptive transfer of CD4+CD25+ T cells from naïve mice to CD25+ T cell-depleted mice reversed the enhanced disease severity (42). In this context, CD4+ T cells’ response to Salmonella-CFA/I provoked enhanced Th2-type and regulatory cytokines IL-4, IL-10, and TGF-β production, as well as enhanced numbers of FoxP3+CD4+CD25+ T cells. FoxP3 is a functional marker of regulatory T cells and can also be expressed by CD4+CD25− T cells. It has been suggested that FoxP3 directs the conversion of induced regulatory CD4+CD25+ T cells (rev. in 43). CD39, ectonucleoside triphosphate diphosphohydrolase-1, expressed predominantly on FoxP3+ cells, has been shown to limit inflammation via generation of extracellular adenosine and was recently implicated a possible alternative marker for Treg cells (26, 27). Oral immunization with Salmonella-CFA/I was shown to enhance the expression of CD39 by FoxP3+ T cells. In fact, very limited percentages of the FoxP3− T cells in both CD25+ and CD25−T cell subsets were CD39+. These findings led us to question the role of Salmonella-CFA/I-primed CD4+ T cells, including the significance of the both CD25− and CD25+ T cell subsets in suppression of CIA. Flow cytometry analysis for CD4+CD25+ and CD4+CD25−T cells showed co-segregation with FoxP3 and CD39. An adoptive transfer of CD4+, CD4+CD25−, or CD4+CD25+ T cells from mice immunized with Salmonella-CFA/I or Salmonella vector was performed to mice previously challenged with CII approximately 7 – 9 days before disease onset. As a result, recipients of total Salmonella-CFA/I-primed CD4+ T cells did not show symptoms of CIA for 10 days when compared to control diseased group. Although within the subsequent week, the incidence of CIA in this group achieved 100% as did the control group, and the average clinical scores remained remarkably lower. Recipients of isolated CD4+CD25− and CD4+CD25+ T cells demonstrated ~50% less disease frequency and lower average clinical scores per group. Corresponding T cell subsets from Salmonella vector-primed could not reverse CIA; although some reduction in average clinical scores and incidence of the disease was observed. Salmonella-CFA/I-induced CD4+CD25− and CD4+CD25+ T cells were not equally protective in EAE (19). Only partial protection was achieved by treatment with CD4+CD25− T cells, whereas CD4+CD25+ T cells were completely protective. In contrast, CD25− and CD25+ T cell subsets from Salmonella-CFA/I-vaccinated mice showed equivalent protection against CIA, but were suboptimal when compared to protection conferred by combined CD25− and CD25+CD4+ T cells. These results suggest that Salmonella-CFA/I may induce regulatory cells in both subsets, CD4+CD25− and CD4+CD25+ T cells.
We investigated the role of regulatory cytokines IL-4, IL-10, and TGF-β in protection by Salmonella-CFA/I. In vitro experiments demonstrated that all three cytokines mediate suppression of CII-specific CD4+ T cell proliferation by CFA/I-specific CD4+ T cells. Previous studies have shown IL-4 to promote the development of regulatory CD4+CD25+ T cells (44 and our observations). In this current study, FoxP3 expression was reduced by 50% when IL-4, IL-10, or TGF-β was neutralized.
The relevance of TGF-β was further implicated when anti-TGF-β mAb was administered in vivo to recipients of Salmonella-CFA/I-primed CD4+ T cells and nearly completely neutralized the protective effect by the adoptively transferred CD4+ T cells. While anti-IL-4 mAb treatment did not completely neutralize the protective properties of the adoptively transferred CD4+ T cells, it did essentially compromise protection, resulting in disease incidence to be similar to the PBS control group, and it significantly increased clinical scores for those treated mice. Clearly these experiments provide evidence of the importance of TGF-β and IL-4 in suppression of inflammation by Salmonella-CFA/I.
Weakened CIA in recipients could also have been attributed to depressing IL-17 production by CII-reactive CD4+ T cells. IL-17 produced by Th17 cells (45, 46), distinct from Th1 and Th2 cell populations, has been shown to play an important role in CIA (46, 47). Significant acceleration of CIA onset was achieved after i.v. IL-17 gene transfer, and if IL-17 was over expressed in the joint cavity, aggravated cartilage and bone destruction were observed (48). Recently Röhn T.A. et al. succeeded in inhibiting the development of CIA, Ab-induced arthritis, as well as suppressing EAE by immunization against IL-17 prior to induction of diseases (49). In this study, IL-17 suppression was mediated by Salmonella-CFA/I-primed CD4+ T cells, producing Th2-type and regulatory cytokines, as also shown in treating EAE (18).
Moreover, IL-27 was modestly depressed in Salmonella-CFA/I-vaccinated mice. IL-27 has been shown to enhance IFN-γ production by naive CD4+ T cells and NK cells (50), and mice lacking the IL-27 receptor showed reduced Th1 cells (51). Although such findings suggest that IL-27 maybe Th1 cell-polarizing, there are opposing reports describing such role in different rodent arthritis models. In a rat adjuvant-induced arthritis model, treatment with a DNA construct expressing murine IL-27 induced a rat anti-IL-27 Ab response neutralizing rat IL-27 resulting in significantly diminished disease (52). Likewise, treatment with an anti-IL-27p28 Ab mimicked this protective effect (52). Using a proteoglycan-induced arthritis model, IL-27 receptor−/− mice showed reduced disease incidence and clinical scores that correlated with reduced IFN-γ and enhanced IL-4 (53). In contrast, mice with CIA treated with recombinant IL-27 at the onset of disease showed reduced disease incidence and clinical scores that correlated with reduced IL-6 and IL-17 (54). In our study, both IL-6 and IL-17 were diminished as IL-27 in Salmonella-CFA/I-vaccinated mice that correlated to the increased production of regulatory cytokines. Perhaps the increased production of IFN-γ relative to PBS-treated mice was in part sustained by the residual IL-27. Additional studies are planned to address such a possibility and determine whether IL-27 has a Th1-type-promoting effect in our system.
In conclusion, we have shown that oral immunization with Salmonella-CFA/I, a vaccine originally designed to protect against a diarrheal disease, is capable of inhibiting systemic inflammation in the CIA model causing development of Th2-type and TGF-β-producing CD4+ T cells. The contribution of vaccine-induced CD4+CD25− and CD4+CD25+ regulatory T cells and possibly CD39+ regulatory cells in such protection is clearly demonstrated in experiments with adoptive transfer of these subsets to mice in which CIA was induced. Neutralizations of TGF-β compromised protection against CIA in recipients of Salmonella-CFA/I-primed CD4+ T cells, and neutralization of IL-4 significantly weakened the protective properties of transferred cells. Salmonella-CFA/I-primed CD4+ T cells producing regulatory cytokines IL-4, IL-10, and TGF-β modulated the Th cell response to CII in recipient mice, further promoting the production of regulatory cytokines and suppressing IFN-γ and IL-17 production.
Acknowledgments
We thank Ms. Nancy Kommers for her assistance in preparing this manuscript.
Footnotes
This work is supported by U.S. Public Health Service Grant AT-04312 and AI-41123 and in part by Montana Agricultural Station and U.S. Department of Agriculture Formula Funds. The VMB flow cytometry facility was, in part, supported by NIH/National Center for Research Resources, Centers of Biomedical Excellence P20 RR-020185, and an equipment grant from the M.J. Murdock Charitable Trust.
Abbreviations used in this paper: RA, rheumatoid arthritis; CIA, collagen-induced arthritis; CII, collagen II; ETEC, enterotoxigenic E. coli; CFA/I, colonization factor antigen/I; EAE, experimental autoimmune encephalomyelitis; PLP, proteolipid protein; Treg, regulatory T cells; LN, lymph node; CFC, cytokine-forming cells; MLN, mesenteric LN; HNLNs, head and neck lymph nodes; FoxP3, forkhead box P3;
References
- 1.Sibilia J. Novel concepts and treatments for autoimmune disease: ten focal points. Joint Bone Spine. 2004;71:511–517. doi: 10.1016/j.jbspin.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 2.de Kleer I, Vastert B, Teklentburg G, Arkensteijn G, Yung GP, Albani S, Kuis W, Wulffraat N, Prakken B. Autologous stem cells transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:696–1702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- 3.Clynes R. Immune complexes as therapy for autoimmunity. J Clin Invest. 2005;115:25–27. doi: 10.1172/JCI23994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopman WJ. Prospects for autoimmune disease. Research advances in rheumatoid arthritis. JAMA. 2001;285:648–650. doi: 10.1001/jama.285.5.648. [DOI] [PubMed] [Google Scholar]
- 5.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould DJ, Bright C, Chernajovsky Y. Inhibition of established collagen-induced arthritis with a tumor necrosis factor-α inhibitor expressed from a self-contained doxycycline regulated plasmid. Arthritis Res Ther. 2004;6:R103–R113. doi: 10.1186/ar1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorbecke GJ, Shan R, Leu CH, Kuruvilla AP, Hardison AM, Palladino MA. Involvement of endogenous tumor necrosis factor α and transforming growth factor β during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb AB. Clinical research helps elucidate the role of tumor necrosis factor-α in the pathogenesis of T1-mediated immune disorders: use of targeted immunotherapeutics as pathogenic probes. Lupus. 2003;12:190–194. doi: 10.1191/0961203303lu354xx. [DOI] [PubMed] [Google Scholar]
- 9.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunization against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 10.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- 11.Terato K, Haeper DS, Griffiths MM, Hasty DL, Ye XJ, Cremer MA, Seyer JM. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmun. 1995;22:137–147. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- 12.Levine MM, Tacket CO, Sztein MB. Host-Salmonella interaction: human trials. Microbes Infect. 2001;3:1271–1279. doi: 10.1016/s1286-4579(01)01487-3. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Pascual DW, VanCott JL, McGhee JR, Maneval DR, Jr, Levine MM, Hone DM. Immune responses to novel Escherichia coli and Salmonella typhimurium vectors that express colonization factor antigen I (CFA/I) of enterotoxigenic E. coli in the absence of the CFA/I positive regulator cfaR. Infect Immun. 1995;63:4933–4938. doi: 10.1128/iai.63.12.4933-4938.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual DW, Hone DM, Hall S, van Ginkel FW, Yamamoto M, Walters N, Fujihashi K, Powell RJ, Wu S, Vancott JL, Kiyono H, McGhee JR. Expression of recombinant enterotoxigenic Escherichia coli colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect Immun. 1999;67:6249–6256. doi: 10.1128/iai.67.12.6249-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: immune responses and vaccines. Vet J. 2001;161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 16.Pascual DW, Trunkle T, Sura J. Fimbriated Salmonella enterica serovar Typhimurium abates initial inflammatory responses by macrophages. Infect Immun. 2002;70:4273–4281. doi: 10.1128/IAI.70.8.4273-4281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters N, Trunkle T, Sura M, Pascual DW. Enchanced immunoglobulin A response and protection against Salmonella enterica serovar Typhimurium in the absence of substance P receptor. Infect Immun. 2005;73:317–324. doi: 10.1128/IAI.73.1.317-324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun S, Gilmore W, Callis G, Rynda A, Haddad A, Pascual DW. A live diarrheal vaccine imprints a Th2 cell bias and acts as an anti-inflammatory vaccine. J Immunol. 2005;175:6733–6740. doi: 10.4049/jimmunol.175.10.6733. [DOI] [PubMed] [Google Scholar]
- 19.Ochoa-Repáraz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol. 2007;178:1791–1799. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brand DD, Kang AH, Rosloniec EF. The mouse model of collagen-induced arthritis. Methods Mol Med. 2004;102:295–312. doi: 10.1385/1-59259-805-6:295. [DOI] [PubMed] [Google Scholar]
- 21.Malmström V, Kjellén P, Holmdahl R. Type II collagen in cartilage evokes peptide-specific tolerance and skews the immune response. J Autoimmun. 1998;11:213–221. doi: 10.1006/jaut.1998.0198. [DOI] [PubMed] [Google Scholar]
- 22.Schramm C, Kriegsmann J, Protschka M, Huber S, Hansen T, Schmitt E, Galle PR, Blessing M. Susceptibility to collagen-induced arthritis is modulated by TGF-β responsiveness of T cells. Arthritis Res Ther. 2004;6:R114–R119. doi: 10.1186/ar1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res Ther. 2004;6:R256–R263. doi: 10.1186/ar1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rynda A, Maddaloni M, Mierzejewska D, Ochoa-Repáraz J, Maœlanka T, Crist K, Riccardi C, Barszczewska B, Fujihashi K, McGhee JR, Pascual DW. Low-dose tolerance is mediated by the M cell ligand, reovirus protein σ1. J Immunol. 2008;180:5187–5200. doi: 10.4049/jimmunol.180.8.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart JM, Cremer MA, Townes AS, Kang AH. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982;155:1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 28.Ascón MA, Hone DM, Walters N, Pascual DW. Oral immunization with a Salmonella typhimurium vaccine vector expressing recombinant enterotoxigenic Escherichia coli K99 fimbriae elicits elevated antibody titers for protective immunity. Infect Immun. 1998;66:5470–5476. doi: 10.1128/iai.66.11.5470-5476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ascón AM, Ochoa-Repáraz J, Walters N, Pascual DW. Partially assembled K99 fimbriae are required for protection. Infect Immun. 2005;73:7274–7280. doi: 10.1128/IAI.73.11.7274-7280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis. Clin Exp Immunol. 1997;111:521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malmström V, Michaëlsson E, Burkhardt H, Mattsson R, Vuorio E, Holmdahl R. Systemic versus cartilage-specific expression of a type II collagen-specific T-cell epitope determines the level of tolerance and susceptibility to arthritis. Proc Natl Acad Sci USA. 1996;93:4480–4485. doi: 10.1073/pnas.93.9.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Berg WB, Joosten LA, Helsen M, van de Loo FA. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol. 1994;95:237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart JM, Dixon FJ. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983;158:378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima H, Hiyama Y, Takamori H, Tsukada W. Cell-mediated transfer of collagen-induced arthritis in mice and its application to the analysis of the inhibitory effects of interferon-gamma and cyclophosphamide. Clin Exp Immunol. 1993;92:328–335. doi: 10.1111/j.1365-2249.1993.tb03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luross JA, Heaton T, Hirst TR, Day MJ, Williams NA. Escherichia coli heat-labile enterotoxin B subunit prevents autoimmune arthritis through induction of regulatory CD4+ T cells. Arthritis Rheum. 2002;46:1671–1682. doi: 10.1002/art.10328. [DOI] [PubMed] [Google Scholar]
- 36.Yoshino S, Sasatomi E, Mori Y, Sagai M. Oral administration of lipopolysaccharide exacerbates collagen-induced arthritis in mice. J Immunol. 1999;163:3417–3422. [PubMed] [Google Scholar]
- 37.Honda A, Ametani A, Matsumoto T, Iwaya A, Kano H, Hachimura S, Ohkawa K, Kaminogawa S, Suzuki K, Sercarz EE, Kumar V. Vaccination with an immunodominant peptide of bovine type II collagen induces and anti-TCR response, and modulates the onset and severity of collagen-induced arthritis. Int Immunol. 2004;16:737–745. doi: 10.1093/intimm/dxh075. [DOI] [PubMed] [Google Scholar]
- 38.Finnegan AC, Kaplan D, Cao Y, Eibel H, Glant TT, Zhang J. Collagen-induced arthritis is exacerbated in IL-10-deficient mice. Arthritis Res Ther. 2002;5:R18–R24. doi: 10.1186/ar601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-γ receptor - deficient mice. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 40.Myers LK, Tang B, Stuart JM, Kang AH. The role of IL-4 in regulation of murine collagen-induced arthritis. Clin Immunol. 2002;102:185–191. doi: 10.1006/clim.2001.5162. [DOI] [PubMed] [Google Scholar]
- 41.Kakimoto K, Katsuki M, Hirofuji T, Iwata H, Koga T. Isolation of T cell line capable of protecting mice against collagen-induced arthritis. J Immunol. 1988;140:78–83. [PubMed] [Google Scholar]
- 42.Morgan ME, Sutmuller RPM, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJM, Snijders A, Offringa R, de Vries RRP, Toes REM. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 43.Fontenot JD, Rudensky A. A well adapted regulatory contrivance: regulatory cells development and the forkhead family transcription factor FoxP3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 44.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. IL-4 receptor α-chain - binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 45.Betelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 46.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 48.Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, van de Loo FA, van den Berg WB. IL-1- independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 49.Röhn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 50.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 52.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–1178. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 53.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 54.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. Interleukin-27 attenuates collagen-induced arthritis. Ann Rheum Dis. 2008;67 doi: 10.1136/ard.2007.083360. In press. [DOI] [PubMed] [Google Scholar]