Abstract
Apparent changes in breeding performance with age measured at the population level can be due to changes in individual capacity at different ages, or to the differential survival of individuals with different capabilities. Estimating the relative importance of the two is important for understanding ageing patterns in natural populations, but there are few studies of such populations in which these effects have been disentangled. We analysed laying date and clutch size as measures of individual performance in a population of mute swans (Cygnus olor) studied over 25 years at Abbotsbury, UK. On both measures of breeding performance, individuals tended to improve up to the age of 6 or 7, and to decline after about the age of 12. Individuals with longer lifespans performed better at all ages (earlier laying, larger clutches) than animals that ceased breeding earlier. We conclude that the apparent mean increase in performance with age in mute swans is due to both individual improvement and differential survival of individuals who perform well, while the decline in older age groups is due to individual loss of function. Our results underline the need to take individual differences into account when testing hypotheses about life histories in wild populations.
Keywords: senescence, reproductive success, mute swan, laying date, clutch size, selective disappearance
1. Introduction
It is well documented that reproductive performance in birds and mammals can vary according to an individual's age or experience (Clutton-Brock 1988; Saether 1990). Typically, individuals improve their breeding performance over their first few breeding attempts; the performance then stabilizes before declining in old age due to senescence (Martin 1995). Saether (1990) examined a large number of studies of birds where juvenile and later age classes were compared; he found that in 91% of 35 species adults laid earlier in the season than juveniles, and in 92% of 48 species adults laid larger clutches than juveniles. This early improvement in mean reproductive success can be explained by a number of different mechanisms (Lack 1966; Forslund & Pärt 1995). First, there may be progressive disappearance of poor-quality breeders (selection hypothesis, Curio 1983) or progressive appearance of good quality breeders (delayed breeding hypothesis; Forslund & Pärt 1995). In these cases of individual heterogeneity, age-specific population trends in breeding performance may differ markedly from individual changes in breeding performance. Hence it is essential, although challenging, to collect data on individually based life histories rather than cross-sectional averages (Vaupel & Yashin 1985). Second, individuals can improve their breeding performances through their experience (e.g. improvement of foraging skills) rather than their age per se (breeding experience hypothesis; Saether 1990). Third, the pattern may result from life-history optimization, where reproductive effort is predicted to increase with age since the residual reproductive value decreases with age (Williams 1966; Charlesworth 1994). Testing this idea of an optimization of individual reproductive effort (restraint hypothesis; Curio 1983) again requires lifelong measures of reproductive effort and success. These hypotheses are not mutually exclusive and several authors have suggested that they act in concert to explain the early improvement in reproductive performance (Forslund & Pärt 1995).
More recently, an increasing number of studies has demonstrated a decline in reproductive output (senescence) after a mid-life period during which performance remains more or less constant (e.g. Newton 1989; Martin 1995; Bennet & Owens 2002; Reid et al. 2003), although there are also some striking counter claims, for example, in common terns Sterna hirundo (Nisbet et al. 2002; but see Gonzalez-Solis et al. 2004) and Leach's storm-petrels Oceanodroma leucorhoa (Mauck et al. 2004) where despite reasonable sample sizes, no evidence for a diminution of performance in older individuals was found. It has often been pointed out that, as with early improvement, an age-related decline in mean reproductive performance measured at the population level can be due either to changes within individuals, or in the quality of individuals that are active at different ages, or to a combination of such effects (Vaupel & Yashin 1985; van de Pol & Verhulst 2006).
Two relatively recent studies have considered the idea that there is a sharp drop in performance in the last year of the life, consistent with the idea that death is due to extrinsic causes such as disease, but that these may act less abruptly than (e.g.) an age-independent source of mortality such as the weather or predation. Coulson & Fairweather (2001) showed that in black legged kittiwakes the last reproductive attempt was significantly less successful than the penultimate one, at whatever age this occurred. Rattiste (2004) demonstrated a similar effect in the common gull (Larus canus), and also showed that the effect introduced an artefact into the relationship between overall age-related breeding success and longevity. Thus, attempts to model age-related breeding success need to take such terminal effects into consideration as well as age.
Longitudinal studies over the whole lifespan that tease these effects apart remain very scarce in both mammals (Gaillard et al. 1994; Nussey et al. 2006) and birds (Cam et al. 2002; Reid et al. 2003). Here, we make use of an exceptional long-term dataset in the long-lived mute swan (Cygnus olor) to investigate within-individual changes with age in reproductive performance (laying date and clutch size) while controlling for potential covariance between individual quality and longevity. Our aim is to assess the relative importance of within and between-individual effects in contributing to cross-sectional age-specific patterns of reproduction.
2. Material and methods
(a) Study species and data collection
Mute swans have been breeding on the Fleet at Abbotsbury, Dorset since at least the thirteenth century. They are unusual in Britain in that they nest in a colony (for a general description, see Perrins & Ogilvie 1981; Perrins et al. 1994). Most of the breeding birds were themselves raised in the colony in earlier years, and since they are all individually ringed, their ages are precisely known (McCleery et al. 2002). In recent years, approximately 150 pairs have bred each year and for each successful breeding attempt, the identity of the parents, the laying date for each clutch (the date on which the first egg is laid by each female) and the clutch size, are known. All pairs that complete a clutch and start to incubate are identified. Emigration from the colony is low and there are no records of this happening after a bird has commenced breeding.
Since we are interested here in age effects including senescence, birds that have not been recorded breeding for 2 consecutive years are considered to be dead, and birds known to be alive in the last 2 years of the study period are excluded from the analysis because their life histories were incomplete. Exact age of death was not known for many of the birds hence in our tests we considered phenotypic disappearance using age at last reproduction rather than longevity (see §2b below).
One obvious variable affecting performance might be the age of the mate. However, in this study population, the age of the male is closely correlated with that of the female, even in many of the older birds which have lost their earlier mate and taken a new one (Perrins & McCleery 1997). This assortative mating by age prevented us from examining the effect of male age on female reproductive performance. Additionally, laying date and clutch size are usually considered as sex-limited characters (see Charmantier et al. (2006b) for justification in this species). Hence, only females are considered in the following analyses.
(b) Statistical analysis
There are several problems involved in analysing longitudinal data to investigate age-dependent trends while controlling for individual quality effects.
(i) Repeated measures
Individuals are measured repeatedly in different years until their death. This violates the assumption that each observation of breeding performance is drawn at random from a population of breeding performances and ages. However, it is possible to separate changes occurring within individuals from those due to changes in the composition of the cohorts breeding at different ages using a statistical model containing a random effect for each individual female. The solution we adopt here is to employ a general linear mixed model (hereafter abbreviated by LMM) using the restricted maximum-likelihood method as implemented in the REML procedure of the program Genstat.
(ii) Choice of parameterization
Three parameters are potentially of interest as explanations for a specific breeding performance, namely the age of the female, the age at which the individual was last known to breed (hereafter called ALR, age at last reproduction), and the number of future potential reproductive attempts she has left to make (hereafter called RRL, residual reproductive lifespan). Since RRL=ALR−age, it is obviously not possible to fit all three parameters in a single model. However, there are several ways to parameterize models predicting reproductive performance from age and breeding lifespan. Bearing in mind the discussion of terminal and age-related effects in §1, the two most interesting here are as follows:
These models have identical deviances but different parameter values. The first asks whether there is a curvilinear relationship between age and breeding performance, and whether, in addition, there is an additive effect of ALR. Fitting interactions between ALR and the age terms tests whether any age effect is different for individuals with different lifespans. The second model poses the question slightly differently, in terms of the time until the individual will stop breeding, rather than its current age. Again, interactions can be added to investigate whether the shape of the curve is different for animals with different breeding lifespans. Owing to the repeated measures problem, and because there are large differences in laying date between years, we need to extend the basic models to:
Although very close, these two models have slightly different deviances because we do not have exactly the same number of females in each age class in each year. The presence of the polynomial terms in age means that the model components must be fitted sequentially due to considerations of marginality. Significance tests for lower-order terms are not meaningful when a higher-order term is present in the model (e.g. Grafen & Hails 2002) so we give only the results of sequentially adding the terms.
(c) Other potential biases and interpretation
If variation of reproductive performance with age results in a combination of improvement early in life and decline later on, the predominant effect should be a quadratic component, and the significance of this parameter is the main test of the hypothesis that the performance of individuals improves early in life and declines at older ages. However, owing to the imbalance of the data, with all individuals in the dataset contributing points for early ages but only relatively few individuals contributing to the decline, there is a danger that a fitted curve indicating a decline in older age groups may in fact depend largely on the shape of the increase in younger age groups. A related problem is the extreme statistical imbalance of a model involving both age and longevity. Obviously, only animals which live a long time can be observed as older individuals. There is, therefore, no completely satisfactory way of modelling these data.
Hence to verify our main conclusions about age effects, we augment the analysis with an extremely conservative and robust model derived as follows. We calculate residuals from an analysis of variance of laying date (or clutch size) by year. As the standard deviations of laydate and clutch size do not differ much between years we use raw rather than standardized residuals so as to retain a scale of measurement of days (or eggs). We then divide the data into age groups (3–6, 7–11, 12 or more) and ALR groups (with the same boundaries). Finally, we compare the mean residualized performance measure for each grouping, using Bonferroni pairwise tests. This is an extremely conservative approach with a very low chance of a type I error, not least because the family wise error rate allows for all possible pairwise tests when we in fact use only those which are meaningful. Thus, if we find that the oldest age group has a significantly lower performance we can be extremely confident that this is a real effect.
To verify our conclusions about RRL effects, we use a simpler but equally robust analysis. Residuals for laying date (or clutch size) are calculated as above. We then perform a matched pair's t-test for each individual on their last and penultimate breeding attempt. Although all birds must by definition breed at ALR, some may have failed to breed successfully in the year before last reproduction, so these individuals were dropped from this analysis.
3. Results
The full dataset consists of 2020 breeding attempts by 459 individual female swans between 1979 and 2003. The median number of breeding attempts by individuals is three, with an inter-quartile range of two to six. Approximately 30% of the individuals (141) bred for the first time when aged 3 and a further 35% (162) bred for the first time when aged 4. Approximately 10% did not enter the breeding population until the age of 7 or later (Charmantier et al. 2006c). As the statistical models included a term for age of last reproduction we eliminated 587 breeding attempts by 126 individuals who bred subsequently to 2003 from the analysis.
(a) Laying date
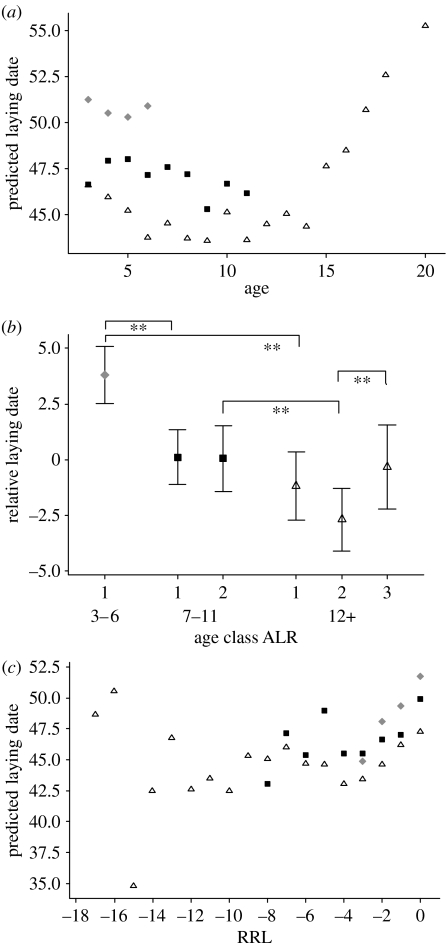
There are large between-year differences in the timing of the season; the earliest annual mean laying date was 7 April 1988, while the latest was 29 April 1983, justifying the inclusion of year as a fixed effect. After correcting for year effects, powers of age up to the cubic are highly significant predictors of laying date (table 1a). The quadratic term in age in particular is highly significant and positive, reflecting a concave up relationship. Thus, birds of intermediate age tend to lay relatively earlier than birds of early or late age (figure 1a). The term for ALR is significantly negative, showing that at any age, birds which will live longer tend to lay earlier than those that have a shorter lifespan (figure 1a), as shown also in figure 1b where the class of individuals who did not breed after age 6 performed much worse than the other age groups and longevity classes (all pairwise tests significant at an adjusted p<0.005). The interaction between age and ALR is significant, reflecting the fact that improvement in early ages is stronger for long-lived birds (ALR=12+) than shorter-lived birds (ALR=7–11), as illustrated by the confirmatory analysis in figure 1b. The interaction between ALR and age2 is also significant and positive, suggesting that the relationship is more concave for the birds which live the longest, but the interaction between ALR and age3 was not significant (table 1a).
Table 1a.
The effects of age of breeding, ALR and their interaction on female mute swan laying date. (Results from a LMM with female identity as a random effect (model 1). The test statistics are given for the full model, fitting the terms sequentially as explained in the text.)
| fixed term | Wald statistic | d.f. | Wald/d.f. | Χ2p-value | estimate | s.e. |
|---|---|---|---|---|---|---|
| year | 481.83 | 24 | 20.08 | <0.001 | ||
| age | 5.39 | 1 | 5.39 | 0.020 | −8.18 | 1.39 |
| age2 | 68.35 | 1 | 68.35 | <0.001 | 0.854 | 0.176 |
| age3 | 7.47 | 1 | 7.47 | 0.006 | −0.026 | 0.007 |
| ALR | 30.19 | 1 | 30.19 | <0.001 | −0.837 | 0.146 |
| ALR×age | 10.53 | 1 | 10.53 | 0.001 | −0.441 | 0.132 |
| ALR×age2 | 6.69 | 1 | 6.69 | 0.010 | 0.023 | 0.009 |
Figure 1.
(a) Fitted values for the LMM exploring age effects on laying date (model 1), illustrated with three ALR classes; 3–6 years, grey diamonds; 7–11, black squares; 12 or more, open triangles. Note that for illustrative purposes the fitted values for the continuous variable ALR are averaged across the three classes to give three curves representing ALR. Owing to different numbers of individuals in each age/ALR class, the effects of different years and the effects of individuals, the averaged points do not appear as continuous curves. (b) Mean and Bonferroni CIs for the residualized laying date for six age/ALR classes. Since there are 15 possible comparisons, the Bonferroni α-level is set to 0.05/15=0.003 for each test, and the CIs are 99.7% CIs. Standard errors are pooled standard errors, and the d.f. for the tests are 2008. In fact, only seven meaningful comparisons are considered (age classes across ALR groups (four) and age differences within age classes (three)). Horizontal bars show significant differences with an indication of the Bonferroni adjusted significance of the difference (*p<0.05, **p<0.005). Symbols are the same as given in (a). (c) Fitted values for the LMM exploring effects of RRL on laying date (model 2), illustrated with three ALR classes. Note that RRL is plotted from right to left, so that last reproduction occurs at the r.h.s. Symbols are the same as given in a.
As with model 1, model 2 on laying date shows a concave up relationship with the time until last breeding (RLL), with significant linear, quadratic and (just) cubic coefficients (table 1b). The fitted values for this model are shown in figure 1c, which shows that, notwithstanding the significant ALR×RRL2 term which implies different curvatures depending on longevity, in all age classes laying date appears to become later in the last three or possibly four breeding attempts, regardless of the age at which breeding ceases. The final diminution in performance is confirmed by a matched pairs t-test on the ultimate and penultimate years of breeding which reveals a significant difference for individuals with ALR>6 years, that is, relative laying date is delayed in their last year of breeding (matched pairs t: mean difference=1.98 days, s.e.=0.99, t135=2.00, p=0.047). Note, however, that this does not test the claim that the decline takes place over more than just the last 2 years. Further detailed work is needed to fully tease this apart.
Table 1b.
The effects of RRL, ALR and their interaction on female mute swan laying date; results from an LMM with female identity as a random effect (model 2).
| fixed term | Wald statistic | d.f. | Wald/d.f. | Χ2p-value | estimate | s.e. |
|---|---|---|---|---|---|---|
| year | 478.55 | 24 | 19.94 | <0.001 | ||
| RRL | 11.48 | 1 | 11.48 | <0.001 | −1.61 | 0.46 |
| RRL2 | 11.69 | 1 | 11.69 | <0.001 | 0.193 | 0.104 |
| RRL3 | 3.98 | 1 | 3.98 | 0.046 | 0.007 | 0.004 |
| ALR | 25.36 | 1 | 25.36 | <0.001 | −1.194 | 0.143 |
| ALR×RRL | 52.51 | 1 | 52.51 | <0.001 | −0.062 | 0.080 |
| ALR×RRL2 | 7.34 | 1 | 7.34 | 0.007 | −0.025 | 0.009 |
(b) Clutch size
We used the same type of LMMs to investigate age effects on clutch size, with female identity included as a random effect. Mean clutch size has considerable annual variation, ranging from a mean of 4.07 in 1982 to 6.33 in 1999, so year was again added as a fixed effect. We also added laying date as a covariate because in this species, as in many others, clutch size is strongly negatively correlated with laying date (table 2a; Charmantier et al. 2006b). Hence, the patterns reported here are independent of the temporal dependence of clutch size within years.
Table 2a.
Results from LMM model 1 on clutch size.
| fixed term | Wald statistic | d.f. | Wald/d.f. | Χ2p-value | estimate | s.e. |
|---|---|---|---|---|---|---|
| year | 197.15 | 24 | 8.21 | <0.001 | ||
| laying date | 441.59 | 1 | 441.59 | <0.001 | −0.071 | 0.004 |
| age | 28.47 | 1 | 28.47 | <0.001 | 0.956 | 0.170 |
| age2 | 34.91 | 1 | 34.91 | <0.001 | −0.85 | 0.019 |
| age3 | 11.41 | 1 | 11.41 | <0.001 | 0.002 | 0.0006 |
| ALR | 5.31 | 1 | 5.31 | 0.021 | 0.059 | 0.020 |
| ALR×age | 3.57 | 1 | 3.57 | 0.059 | 0.010 | 0.005 |
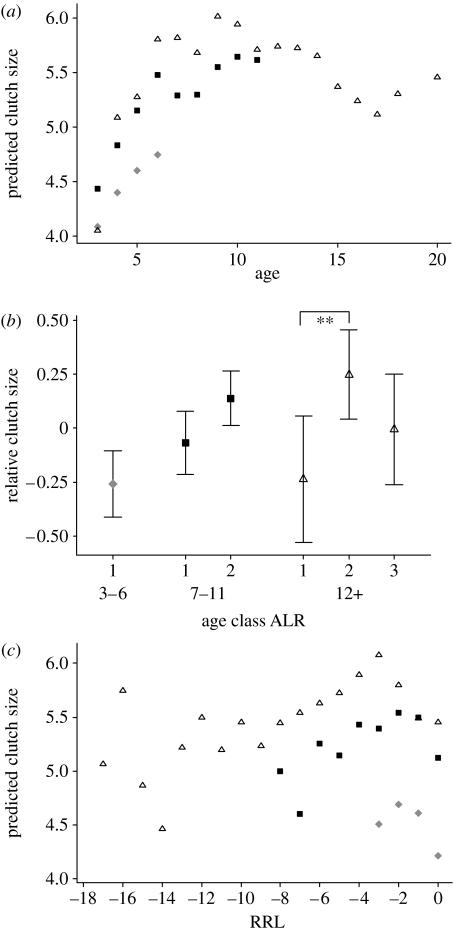
The quadratic term in age is significantly negative (table 2a), so the relationship between clutch size and age is concave down (figure 2a). As with laying date, the early improvement in clutch size is stronger (and significant) in the longest lived birds (figure 2b). The significant and positive effect of ALR means that birds that live longer tend to lay more eggs at all ages, as illustrated in figure 2a (although this was not supported by the confirmatory analysis, see figure 2b and associated t-tests, all pairwise differences are not different from 0). The oldest age classes seem to show a decline in clutch size at the oldest ages, for example, birds that will live to 15 show a decline in clutch size after approximately 13. However, the senescence in clutch size is relatively weak after controlling for laying date, as shown by the non-significant difference between the two age classes of long-lived birds in figure 2b (difference=−0.166, s.e.=0.0955, t=1.74, n.s.).
Figure 2.
(a) Fitted values for the LMM exploring age effects on clutch size illustrated with three ALR classes. Symbols are the same as given in figure legend 1. (b) Mean and Bonferroni CI for the residualized clutch size for six ALR classes. See legend of figure 1b. The difference between age classes 7–11 and age classes 3–6 for ALR group, 7–11 is not significant (t=2.68, adjusted p=0.11) and is considerably less than the equivalent difference in the ALR=12+ group (t=3.67, adjusted p=0.004). Symbols are the same as given in figure legend 1. (c) Fitted values for the LMM exploring effects of RRL on clutch size illustrated with three ALR classes. Symbols are the same as given in figure 1a.
In model 2 for clutch size, the dominant component is the squared term in RRL, predicting that clutch size rises to a peak at about RRL=−4 years before declining. The confirmatory analysis focuses on the final decline and shows that birds lay fewer eggs (adjusted for laying date and year) in their last year of breeding, provided they breed beyond the age of 6 (matched pairs t-test mean difference=−0.320, s.e.=0.147, t134=2.18, p=0.031), but, as with model 2 for laydate, the fitted values for clutch size (figure 2c) suggest a decline occurring over more than the last attempt. The interaction ALR×RRL2 is not significant (and is therefore not shown in table 2b), indicating that the curvature is not detectably different in different age classes.
Table 2b.
Results from LMM model 2 on clutch size.
| fixed term | Wald statistic | d.f. | Wald/d.f. | Χ2p-value | estimate | s.e. |
|---|---|---|---|---|---|---|
| year | 193.18 | 24 | 8.05 | <0.001 | ||
| laying date | 435.46 | 1 | 435.46 | <0.001 | −0.071 | 0.003 |
| RRL | 0.69 | 1 | 0.69 | 0.405 | 0.187 | 0.060 |
| RRL2 | 10.02 | 1 | 6.95 | 0.008 | −0.043 | 0.012 |
| RRL3 | 1.65 | 1 | 1.65 | 0.199 | 0.0011 | 0.0006 |
| ALR | 30.45 | 1 | 30.45 | <0.001 | 0.148 | 0.016 |
| ALR×RRL | 20.47 | 1 | 20.47 | <0.001 | 0.026 | 0.005 |
4. Discussion
The long-term monitoring of individual swans has allowed us to demonstrate here that variation in reproductive performance, across ages is the result of two independent processes: (i) age-specific individual variation, that is, improvement followed by senescence within individuals and (ii) effects of individual quality persistent throughout their life.
Most female mute swans start breeding at 3 or 4 years old and performance on both measures improves (i.e. laying gets earlier and clutches get larger) until around the age of 7. This within-individual improvement in the first few years of life has previously been attributed to older, and thus more experienced birds feeding more efficiently or acquiring better territories in which more food is available (breeding experience hypothesis; Saether 1990; Martin 1995). However, this does not seem a particularly plausible argument for the Abbotsbury swans, which nest colonially and are not dependent on their territories for food. Furthermore, much food is provided for them, artificially, in the run-up to breeding. Possibly in the melée at feeding times, the younger birds are not able to obtain as much food as the older ones, but the food is scattered quite widely and it is not clear that competition of this sort is occurring. A more likely explanation in the context of a colonial nesting habit is a greater susceptibility to social interference in younger birds. Preliminary evidence (Charmantier 2005, unpublished data) suggests that young birds are more likely to lose confrontations than older birds, and that they may spend more time fighting early in the season, which may delay their laying. Exactly how this might affect clutch size, in view of the evidence that this varies with age after allowing for differences in laying date, is less clear, but will require detailed behavioural observation.
We have also revealed individual reproductive senescence, with a decline in individual performance after the age of 12–13 years (figures 1a and 2a). By statistically eliminating laying date effects, we highlighted late age effects on clutch size over and above those due to differences in timing of breeding, although the clutch size decline appears weaker than the senescence in laying date (figures 1b and 2b). Demonstrating senescence in birds, and especially reproductive senescence, has proven a challenge because very few birds reach old ages in the wild and obtaining repeated measures of performance is rarely possible (Ricklefs 2000; Bennet & Owens 2002). Hence, while senescence in birds was first suggested by cross-sectional analyses 30 years or so ago (Perrins & Moss 1975; Dhondt 1985), unbiased evidence for within-individual senescence in longitudinal studies is very recent and still scarce (Reid et al. 2003; Catry et al. 2006; van de Pol & Verhulst 2006; Brommer et al. 2007). By analysing the decline in performance in terms of RRL as well as age, we have a suggestion that terminal decline occurs in a consistent way at different ages. While previous work (Coulson & Fairweather 2001; Rattiste 2004) has focused on the last breeding attempt our results seem to show the decline occurring over more than the last year of reproduction. As far as we know, this has not been seen before. Further work on this, including measurements of fitness as well as reproductive effort, is in progress.
If we can detect changes in RRL-related behaviour in this way it is possible, at least in principle, that the system could have some information about its approaching demise, and therefore throw everything into a last reproductive effort. Whether this makes evolutionary sense depends on the premise that increasing current reproductive effort reduces survival to the next season, for which we have no evidence here, but in any case the data strongly suggest that the reverse is true—last reproductive effort appears to be less (eggs laid later and fewer eggs after adjusting for laying date) rather than more. This idea does not seem relevant in this study.
One peculiarity of this mute swan population that might have facilitated the detection of senescence in laying date and clutch size in such a clear way is its semi-wildness. Swans in Abbotsbury are fed twice a day, which probably diminishes the hazards of food foraging compared with wild birds, and it is well known that protected populations display stronger senescence (Rose 1991) owing to the persistence of lower-quality birds which would not have survived in harsher conditions. There is no clear evidence that this is the case at Abbotsbury compared with other populations in the UK, though comparisons are difficult as many swan populations receive informal supplements from members of the public. However, a study on the Upper Thames (Reynolds 1972), where many territories are well away from areas frequented by the public, shows relationships between clutch size and laying date, and an improvement in performance by older individuals, essentially identical to those observed for Abbotsbury. This implies that these responses are not determined by extrinsic factors such as food supply, and must therefore be intrinsic properties of the individual animals. If this interpretation is correct, it follows that the observed age-related changes in behaviour must be due to some age-related changes in the neuroendocrine mechanisms relating day length, differences between years (presumably the weather) and egg laying. In addition it suggests that the loss of competence as the end of life approaches is itself subject to some individual variation, either in intrinsic quality or in exposure to factors acting over some time, such as chronic disease.
Whether or not the food supplementation makes it easier to detect it, the clear evidence for strong senescence opens new perspectives of research in wild and semi-wild populations with known pedigrees, as it suggests that we will be able to test models of senescence evolution, for example, by investigating age-specific genetic variances and covariances outside the classic laboratory models and conditions (Charmantier et al. 2006a; Brommer et al. 2007).
The positive effect of ALR on performance at all ages shows that birds of consistently lower-quality (i.e. later breeders with smaller clutches) are dropping out of the population sooner (van de Pol & Verhulst 2006). This is a typical case of phenotypic disappearance that would strongly bias the interpretation of age-related changes in a cross-sectional study. In fact, when we come to consider the improvement in performance with age measured on the population as a whole there is an effect due to differential survival of higher-quality individuals, as well as a within-individual age effect for birds of any given quality. This result is also consistent with earlier findings in this colony showing the phenotypic correlation between age at first breeding and ALR is less positive than the null expectation (Charmantier et al. 2006c), meaning that higher-quality birds were able to breed both relatively early and relative late in their lives.
We have shown here that selective disappearance of phenotypes highlighted by the inclusion in our models of ALR can be of great importance in age-related performance. This suggests that studies of age-specific mortality, and especially actuarial senescence, should also take into account this individual heterogeneity (Vaupel & Yashin 1985; Cam et al. 2002). An interesting avenue once both reproductive and actuarial senescence are adequately modelled is to relate the two and especially test whether the two types of senescence start at the same age and are correlated in their rate within individuals.
The causes of individual heterogeneity such as that shown here are, at present, poorly understood for most populations. Previous work on this population suggests genetic variation for age-specific reproductive trajectories (i.e. in the shape of age-specific patterns) manifested as a genetic correlation between age at first and last reproduction (Charmantier et al. 2006c), but the effects shown in this paper are additional to those, influencing the amount of reproduction across the entire lifespan. While a range of contributory effects, including additive genetic, non-additive genetic and permanent environmental effects have been demonstrated in some populations (e.g. Coulson et al. 2006; Nussey et al. 2006; Foerster et al. 2007), simultaneous estimation of these effects while correcting for individual variation in age-specific reproductive trajectories will prove quite challenging.
Acknowledgments
This paper was written by Robin McCleery who died suddenly on 16 January 2008; his coauthors dedicate it to his memory. We thank Charlotte Townshend for allowing this study on swans, as well as all the staff and volunteers of the swannery who have collected data over the years, especially David Wheeler and Steve Groves. Remarks from three anonymous referees have greatly improved the manuscript. A.C. was funded by a Marie-Curie Intra-European Fellowship and a Biotechnology and Biological Sciences Research Council (BBSRC) grant to B.C.S. and LEB Kruuk.
References
- Bennet P.M, Owens I.P.F. Oxford University Press; New York, NY: 2002. Evolutionary ecology of birds—life histories, mating systems, and extinction. [Google Scholar]
- Brommer J.E, Wilson A.J, Gustafsson L. Exploring the genetics of aging in a wild passerine bird. Am. Nat. 2007;170:643–650. doi: 10.1086/521241. doi:10.1086/521241 [DOI] [PubMed] [Google Scholar]
- Cam E, Link W.A, Cooch E.G, Monnat J.Y, Danchin E. Individual covariation in life-history traits: seeing the trees despite the forest. Am. Nat. 2002;159:96–105. doi: 10.1086/324126. doi:10.1086/324126 [DOI] [PubMed] [Google Scholar]
- Catry P, Phillips R.A, Phalan B, Croxall J.P. Senescence effects in an extremely long-lived bird: the grey-headed albatross Thalassarche chrysostoma. Proc. R. Soc. B. 2006;273:1625–1630. doi: 10.1098/rspb.2006.3482. doi:10.1098/rspb.2006.3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. 2nd edn. Cambridge University Press; Cambridge, UK: 1994. Evolution in age-structured populations. [Google Scholar]
- Charmantier A, Perrins C, McCleery R.H, Sheldon B.C. Age-dependent genetic variance in a life-history trait in the mute swan. Proc. R. Soc. B. 2006a;273:225–232. doi: 10.1098/rspb.2005.3294. doi:10.1098/rspb.2005.3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmantier A, Perrins C, McCleery R.H, Sheldon B.C. Evolutionary response to selection on clutch size in a long-term study of the mute swan. Am. Nat. 2006b;167:453–465. doi: 10.1086/499378. doi:10.1086/499378 [DOI] [PubMed] [Google Scholar]
- Charmantier A, Perrins C, McCleery R.H, Sheldon B.C. Quantitative genetics of age at reproduction in wild swans: support for antagonistic pleiotropy models of senescence. Proc. Natl Acad. Sci. USA. 2006c;103:6587–6592. doi: 10.1073/pnas.0511123103. doi:10.1073/pnas.0511123103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. University of Chicago Press; Chicago, IL: 1988. Reproductive success. [Google Scholar]
- Coulson J.C, Fairweather J.A. Reduced reproductive performance in the black-legged kittiwake: senescence or terminal illness? J. Avian Biol. 2001;32:146–152. doi:10.1034/j.1600-048X.2001.320207.x [Google Scholar]
- Coulson T, Benton T.G, Lundberg P, Dall S.R.X, Kendall B.E, Gaillard J.M. Estimating individual contributions to population growth: evolutionary fitness in ecological time. Proc. R. Soc. B. 2006;273:547–555. doi: 10.1098/rspb.2005.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curio E. Why do young birds reproduce less well? Ibis. 1983;125:400–404. [Google Scholar]
- Dhondt A.A. Do old great tits forego breeding. Auk. 1985;102:870–872. [Google Scholar]
- Foerster K, Coulson T, Sheldon B.C, Pemberton J.M, Clutton-Brock T.H, Kruuk L.E.B. Sexually antagonistic genetic variation for fitness in red deer. Nature. 2007;447:1107–1110. doi: 10.1038/nature05912. doi:10.1038/nature05912 [DOI] [PubMed] [Google Scholar]
- Forslund P, Pärt T. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. doi:10.1016/S0169-5347(00)89141-7 [DOI] [PubMed] [Google Scholar]
- Gaillard J.M, Allaine D, Pontier D, Yoccoz N.G, Promislow D.E.L. Senescence in natural-populations of mammals—a reanalysis. Evolution. 1994;48:509–516. doi: 10.1111/j.1558-5646.1994.tb01329.x. doi:10.2307/2410110 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Solis J, Becker P.H, Jover L, Ruiz X. Individual changes underlie age-specific pattern of laying date and egg-size in female common terns (Sterna hirundo) J. Ornithol. 2004;145:129–136. doi:10.1007/s10336-004-0023-z [Google Scholar]
- Grafen A, Hails R. Oxford University Press; Oxford, UK: 2002. Modern statistics for the life sciences. [Google Scholar]
- Lack D. Oxford University Press; London, UK: 1966. Population studies of birds. [Google Scholar]
- Martin K. Patterns and mechanisms for age-dependent reproduction and survival in birds. Am. Zool. 1995;35:340–348. [Google Scholar]
- Mauck R.A, Huntington C.E, Grubb T.C. Age-specific reproductive success: evidence for the selection hypothesis. Evolution. 2004;58:880–885. doi: 10.1111/j.0014-3820.2004.tb00419.x. [DOI] [PubMed] [Google Scholar]
- McCleery R.H, Perrins C, Wheeler D, Groves S. Population structure, survival rates and productivity of mute swans breeding in a colony at Abbotsbury, Dorset, England. Waterbirds. 2002;25:192–201. [Google Scholar]
- Newton I. Academic Press; London, UK: 1989. Lifetime reproduction in birds. [Google Scholar]
- Nisbet I.C.T, Apanius V, Friar M.S. Breeding performance of very old common terns. J. Field Ornithol. 2002;73:117–124. [Google Scholar]
- Nussey D.H, Kruuk L.E.B, Donald A, Fowlie M, Clutton-Brock T.H. The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol. Lett. 2006;9:1342–1350. doi: 10.1111/j.1461-0248.2006.00989.x. doi:10.1111/j.1461-0248.2006.00989.x [DOI] [PubMed] [Google Scholar]
- Perrins C.M, McCleery R.H. Pairing behaviour in a colony of mute swans Cygnus olor. Wildfowl. 1997;47:31–41. [Google Scholar]
- Perrins C.M, Moss D. Reproductive rates in great tit. J. Anim. Ecol. 1975;44:695–706. doi:10.2307/3712 [Google Scholar]
- Perrins C.M, Ogilvie M.A. A study of the Abbotsbury mute swans. Wildfowl. 1981;32:35–45. [Google Scholar]
- Perrins C.M, McCleery R.H, Ogilvie M.A. A study of the breeding mute swans Cygnus olor at Abbotsbury. Wildfowl. 1994;45:1–14. [Google Scholar]
- Rattiste K. Reproductive success in presenescent common gulls (Larus canus): the importance of the last year of life. Proc. R. Soc. B. 2004;271:2059–2064. doi: 10.1098/rspb.2004.2832. doi:10.1098/rspb.2004.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M, Bignal E.M, Bignal S, McCracken D.I, Monaghan P. Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol. 2003;72:765–776. doi:10.1046/j.1365-2656.2003.00750.x [Google Scholar]
- Reynolds C.M. Mute swan weights in relation to breeding performance. Wildfowl. 1972;23:111–118. [Google Scholar]
- Ricklefs R.E. Intrinsic aging-related mortality in birds. J. Avian Biol. 2000;31:103–111. doi:10.1034/j.1600-048X.2000.210201.x [Google Scholar]
- Rose M.R. Oxford University Press; New York, NY: 1991. Evolutionary biology of aging. [Google Scholar]
- Saether B.E. Age-specific variation in reproductive performance of birds. Curr. Ornithol. 1990;7:251–283. [Google Scholar]
- van de Pol M, Verhulst S. Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 2006;167:766–773. doi: 10.1086/503331. doi:10.1086/503331 [DOI] [PubMed] [Google Scholar]
- Vaupel J.W, Yashin A.I. Heterogeneity ruses—some surprising effects of selection on population-dynamics. Am. Stat. 1985;39:176–185. doi:10.2307/2683925 [PubMed] [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]