Abstract
Recent work has confirmed that genetic compatibility among mates can be an important determinant of siring success in sperm competition experiments and in free-ranging populations. Most of this work points towards mate choice of less related mates. However, there may also be the potential for mate choice for intermediate or even genetically similar mates to prevent outbreeding depression or hybridization with closely related taxa. We studied relatedness effects on post-copulatory gametic choice and/or sperm competition in an external fertilizer, Peron's tree frog (Litoria peronii), since external fertilizers offer exceptional control in order to test gametic interaction effects on probability of paternity and zygote viability. Sperm competition experiments were done blindly with respect to genetic relatedness among males and females. Thereafter, paternity of offspring was assigned using eight microsatellite loci. Three hybridization trials between L. peronii and a closely related sympatric species Litoria tyleri were also carried out. In the sperm competition trials, males that are more genetically similar to the female achieved higher siring success compared with less genetically similar males. The hybridization trials confirmed that the two species can interbreed and we suggest that the risk of hybridization may contribute to selection benefits for genetically more similar males at fertilization. To our knowledge, this study is the first to show evidence for post-copulatory selection of sperm from genetically more similar individuals within a natural population.
Keywords: sperm competition, genetic compatibility, gamete recognition, cryptic female choice, siring success
1. Introduction
In Maynard-Smith's (1956, 2000) own modest words, ‘I had been working on the effects of inbreeding on fitness … and stumbled over the fact that outbred Drosophila subobscura females reject inbred males’. This ‘stumble’, opened up a new research field in which John Maynard-Smith married ‘inbreeding–outbreeding’ genetics, ethology and sexual selection theory. Since then, researchers have come to appreciate the negative effects on fitness of both inbreeding (mating with ‘too closely’ related individuals) and outbreeding (mating with ‘too distantly’ related individuals).
The causal mechanism to inbreeding depression is believed to be either overdominance (i.e. the increase in the frequency of homozygotes and concomitant decrease in frequency of superior heterozygotes), or through partial dominance (i.e. the increase in homozygosity, and hence expression, of deleterious recessive alleles; Roff 2002). Understanding which of these hypotheses explains most of the variation in inbreeding depression has been the subject of much debate, with support accumulating for both ideas, but with more recent experimental work favouring partial dominance (Roff 2002). Inbreeding depression in the wild was largely believed to be non-existent for a long time, but has recently been repeatedly verified (reviewed in Frankham 1995, 2005), for example, in adder snakes Vipera berus (Madsen et al. 1999) and Darwin's finches Geospiza sp. (Keller et al. 2002), in the latter case only under nutritional constraints.
At the other end of the relatedness continuum, outbreeding, the negative impact on fitness is believed to arise through the breaking up of co-adapted gene complexes, resulting in disruption of local adaptation (Waser et al. 2000), and with a maximal negative effect at hybridization (although this sometimes may lead to introgression of genetic elements with positive fitness effects when backcrossed into the parental species; reviewed in Arnold (1997)). Empirical examples from the wild are mostly represented by plant systems (e.g. Waser et al. 2000), but there are also some examples from the animal kingdom. In ambrosia beetles (Xyleborini), which regularly mate with siblings, outbreeding, but not inbreeding, results in genetic depression (Peer & Taborskyi 2005). A similar example from nematodes demonstrates the importance of selection history on genomic architecture. A comparison between selfing, hermaphroditic Caenorhabditis elegans and its gonochoristic (separate males and females) congeneric Caenorhabditis remanei, showed that the sexual species suffered greatly from inbreeding depression, whereas the selfing C. elegans suffered similar consequences from outbreeding (Dolgin et al. 2007). In rainbow trout (Oncorhynchus mykiss) under experimental conditions, crossings between wild and captive-reared fish result in outbreeding depression (Tymchuk et al. 2007), and in natural populations of the ornate dragon lizard (Ctenophorus ornatus), more outbred females produced offspring with poorer survival (LeBas 2002). At the extreme, the risk of hybridization can potentially be an important selective pressure biasing fertilization towards genetically more similar mates. In frogs, Hoskin et al. (2005) showed that speciation between two different lineages of Litoria genimaculata in a hybrid contact zone results from reinforcement driven directly by natural selection against maladaptive hybridization. However, this has also lead to significant premating isolation of L. genimaculata not only from the other lineage but also from other allopatric populations of its own lineage outside the hybrid zone.
Clearly, both extremes of genetic relatedness will influence phenotypic ‘quality’ of offspring and concomitant lifetime fitness. This predicts stabilizing selection on genomic divergence. Indeed, this has been demonstrated in a natural population of bluegill sunfish (Lepomis macrochirus), in which Neff (2004) found a peak in reproductive success, with a minimum of fluctuating asymmetry, at intermediate genomic divergence. Bridging the gap between genetics and sexual selection theory, Neff's (2004) work also showed that females exercising mate choice produced offspring closer to the intermediate optimum of genomic divergence than would be expected from random mating alone. This also seems to support the classic work by Bateson (1982) on quail, which demonstrates optimal mate choice with respect to outbreeding and an overall preference for first cousins in staged mate choice experiments. As Wright (1933) observed, ‘A certain amount of crossbreeding is favourable but not too much’. Does this mean inbreeding should always be avoided?
Recent theoretical work by Kokko & Ots (2006) strongly suggests inbreeding should not always be avoided, and that an important, overlooked component of mate choice on relatedness is the inclusive fitness benefits it entails and that inbreeding tolerance, for reasons of inclusive fitness and limitations of breeding opportunities, should be remarkably high. In spite of this, they remark that empirical examples of mate choice for close relatives are few, but that some do exist. For example, in frigatebirds (Fregata minor), females actively choose males more related than average but the underlying mechanism remains unknown (Cohen & Dearborn 2004). A similar study of cichlid fish (Pelvicachromis taeniatus) shows that females actively inbreed, resulting in more cooperative parental investment in related than unrelated parents (Thünken et al. 2007). Mate choice in the strongly inbred cestode Schistocephalus solidus is even more remarkable with an overall preference for siblings over any other partner relatedness category (Schjørring & Jäger 2007).
To summarize, as originally outlined by Maynard Smith (1956), parental relatedness seems to be able to drive evolution of mate preferences via its effects on, for example, offspring viability (reinforcement), and can be pronounced under some but not other environmental conditions depending on purging history of detrimental recessives. Thus, we expect heterogeneity in parental genomic compatibility among populations and concomitant variation in how selection shapes genetic partner preferences.
Biased probability of paternity may, however, not only result from mate choice taking place before copulation but also be the result of interactions between egg and sperm, and potentially the female reproductive tract itself in internal fertilizers, resulting in sperm competition or cryptic female choice (e.g. reviews in Birkhead & Pizzari 2002; Neff & Pitcher 2005; Simmons 2005). Previous research on post-copulatory mechanisms has verified biased fertilization success towards males of a particular genotype in chickens, although the underlying mechanism is unknown (Martin & Dziuk 1977). Furthermore, in Drosophila and the sea urchin Echinometra mathaei, experimental work has shown male–female genetic interaction effects with respect to probability of fertilization. Thus, some males have better chances of siring offspring with some females than others (Clark et al. 1999; Palumbi 1999).
In the present study, we designed experiments in order to assess effects of genetic similarity on probability of fertilization in the Australian Peron's tree frog (Litoria peronii; Cogger 2000). Since this is an external fertilizer, we can circumvent the problem of variation in sperm concentration among competing ejaculates by diluting them to the same concentration. This allows us to sort between other potential determinants of probability of paternity, which in the present study include variation in sperm quality among males, fertilization success per se (without sperm competition), and genetic compatibility (Birkhead 1998). Consistent biases in probability of paternity would thus suggest an underlying innate difference in fertilization success, independent of the number of transferred spermatozoa. These results are interpreted in light of the risk for hybridization with the closely related sympatric species, Litoria tyleri. Our rationale for this is that selection for reduced fertilization success for genetically more different males may take effect through male indiscriminate mating behaviour, which is common in both toads and frogs. For example, indiscriminate male mating behaviour is believed to play a role in the formation of hybrids between a number of amphibian species, for example, Rana lessonae and Rana ridibunda hybridize and produce the hybrid species Rana esculenta (Berger 1977; Abt & Reyer 1993; Engeler & Reyer 2001), and Bombina bombina and Bombina variegata hybridize in Central Europe (Szymura 1993; Vörös et al. 2006). Evidence for male indiscriminate mating also comes from the much greater variance in partner traits in male than female túngara frogs Physalaemus pustulosus (Bernal et al. 2007), and the many extreme behaviours anecdotally reported in, for example, European toads (with males that mate with foreign objects, e.g. shoes and tennis balls; M. Olsson 2001, personal observation).
2. Material and methods
We performed our experiments on Peron's tree frogs (L. peronii), since these frogs are readily available and breed in aggregations along the Australian east coast. These frogs are prolonged breeders with the breeding season commencing in late September (Sherman et al. in press). Females then visit the breeding ponds throughout the Australian spring and summer (September–January). Breeding can occur within a variety of habitats but is usually associated with still or slow-flowing bodies of water. Males and females are often found in trees adjacent to slow-flowing rivers, creeks and lagoons, and low-lying areas inundated by summer rains (Cogger 2000; Griffiths 2006). To what extent success in sperm competition is an important fitness component to male L. peronii is unknown, since reproductive success has never been analysed in free-ranging L. peronii. However, on any given breeding night, the operational sex ratio is highly skewed towards males and there is intense competition (often involving physical wrestling) among males for access to females. Multiple males have been observed trying to amplex single females and amplexing pairs are often surrounded by satellite males as they move into the water to spawn. Thus, satellite males are often close enough to amplexing pairs for sperm competition to be a potential selective force in this species (C. D. H. Sherman 2005, personal observation).
Peron's tree frog overlaps in large areas with Tyler's tree frog (L. tyleri). Litoria peronii and L. tyleri are morphologically very similar and have overlapping ranges and breeding seasons (Cogger 2000). Litoria peronii occurs throughout the coastal and inland regions of NSW, southern Queensland and northern Victoria. The range of L. tyleri is restricted to mainly coastal areas of NSW and southern Queensland and occurs completely within that of L. peronii, which has a much more extensive inland range, and there are large areas where this species occurs in allopatry (Cogger 2000). Both species are active during the summer breeding period and can be heard calling from September to January (Cogger 2000; Griffiths 2006).
(a) Field protocol
We conducted sperm competition trials over two field seasons (December 2005 to January 2006 and December 2006 to January 2007). During the first field season, we carried out a pilot study comprising nine sperm competition trials (9 females and 18 males). We then carried out more extensive trials (30 trials) during the second field season (30 females and 60 males). In each year, we collected adult male and female L. peronii from a single site at Darkes Forest, NSW, Australia. This population is considered to be relatively large consisting of more than 600 adult individuals. Only vocalizing males were captured, indicating that they were ready to mate. Gravid female L. peronii were collected during their migration from surrounding vegetation to the breeding pond. Females can easily be distinguished from males by the lack of throat coloration, which is cream to white in females, and bright yellow to grey in males (K. Griffiths & C. D. H. Sherman 2005, personal observation). Frogs were transported to laboratory facilities at the University of Wollongong, where we held males overnight at room temperature, while females were held at 10–12°C to prevent the release of gametes. For each frog, we measured snout–vent length to the nearest millimetre and mass (g) to the nearest 0.01 g. All frogs were used for in vitro fertilization experiments within 24 hours of capture and released back to their place of capture within 72 hours. All work was carried out in accordance with National Parks and Wildlife Services permit S11186 and The Wollongong University animal ethics permits AE04/03-05.
(b) Sperm competition trials
(i) In vitro fertilizations and sperm competition trials in Peron's tree frogs
We followed the protocol for artificial fertilizations outlined by Berger et al. (1994), although our experimental design varied slightly between the two field seasons.
Year 1
Males were induced to shed their sperm into conical tubes (250 ml) after a subcutaneous injection of luteinizing hormone-releasing hormone (Sigma-Aldrich). We injected pairs of randomly chosen males used in competition trials within 2 min of each other to ensure that longevity of sperm did not confound our experiments. Sperm were released into the conical tubes and collected from its apex approximately 2 hours after the hormone injection by gently flushing the conical tube with 4–5 ml of aged water. Sperm concentration for each male was determined using a Hawksley haemocytometer (improved Neubauer, 0.1 mm in depth) and the sperm samples were diluted to equal concentration. Equal volumes of the males’ sperm solutions (1 ml from each male) were then mixed together by gentle pipetting and placed into a single Petri dish. The eggs from a randomly chosen female were harvested by gently squeezing her abdomen directly into a single Petri dish. No females or males were used more than once.
Year 2
Sperm were extracted and standardized in the same manner as in the first year. However, in each sperm competition trial, we included replicates and controls that were not included in the first year. A control Petri dish for each male received sperm only from one male (2 ml) and was used to assess each male's ability to fertilize a female's eggs in the absence of sperm competition. A small aliquot (100 μl) of each male's standardized sperm was taken to determine the proportion of viable sperm within each male's ejaculate using Live/Dead sperm viability kits as per the manufacturer's instructions (Molecular Probes). Three replicate sperm competition Petri dishes were used, and each received a mix of the standardized sperm from the two males (1 ml from each male). Each trial therefore consisted of three replicate sperm competition Petri dishes and two control Petri dishes (one for each male). The eggs from a randomly chosen female were then harvested by gently squeezing her abdomen directly into the five Petri dishes. The eggs were then introduced into the Petri dishes in a haphazard manner with respect to male identity such that any potential order effects were removed. No females or males were used more than once. Approximately, 600 eggs were used in each trial, with an average of 121±21 (s.d.) eggs introduced into each Petri dish.
In both years, after 2–3 min, the egg/sperm mixture was flooded with an additional 100 ml of aged water to avoid eggs drying out as the egg jelly coat expands and absorbs water. After 3 hours, all eggs were transferred into a 750 ml plastic container with aged water and held at a constant temperature of 23°C until hatching.
(c) Hybridization experiment between L. peronii and L. tyleri
In three trials, we investigated fertilization success of L. peronii eggs with sperm from L. tyleri in order to assess, qualitatively, whether there is a risk at all for hybridization between these sympatric congeneric species, and whether hybridization at all constitutes a potential selection pressure. All individuals were collected from the same breeding pond as that previously used for the sperm competition trials. No female L. tyleri were found during these collections, thus the reciprocal crosses using female L. tyleri and male L. peronii could not be carried out.
(d) Assignment of paternity
We collected hatchlings from each sperm competition trial and preserved them in 95% ethanol prior to DNA extraction. In the first year, 44–80 hatchlings per sperm competition trial (mean±s.e., 68±3.88) were collected for the assignment of paternity. In the second year, approximately 20 tadpoles from each of the three replicates in a sperm competition trial (approx. 60 tadpoles in total per trial) were collected for the assignment of paternity (mean per trial±s.e., 61±3.04). A toe clip from each adult in the sperm competition trials was used for DNA extraction and the assignment of paternity. Genomic DNA was isolated from whole tadpoles using Qiagen DNAeasy Tissue Kit as per the manufacturer's instructions. Eight polymorphic microsatellite loci (LP05, LP07, LP08, LP13, LP17, LP19, LP22 and LP23) were used to assign paternity (Sherman & Olsson 2007). Paternity was unambiguously assigned to all offspring (2442 tadpoles over 2 years) according to allele sharing between putative sires, mother and offspring. All loci showed high levels of polymorphism with 15–32 alleles detected per locus. We calculated the probability of identity pID, for increasing locus combinations for our dataset (Waits et al. 2001). This identification estimator calculates the probability that two individuals drawn at random from a population will have the same genotype at multiple loci and is used to access the statistical confidence of the marker system for individual identification. When all eight loci were included, pID was extremely small (2.16×10−13) indicating a high degree of power in estimating genetic similarity among genotypes (Waits et al. 2001).
(e) Statistical analysis
Data from the first year's sperm competition trials were used to test for departures from 50 : 50 siring success between males. In year 2, we used a more sophisticated analysis that included both hatching success in control Petri dishes, and the proportion of viable sperm within each male's ejaculate as main effects on siring success. As these were only scored in year 2, only data from the second field season were included in our final analysis. Genetic similarity among individuals was determined as the proportion of shared alleles (i.e. male–female band sharing; advice and comments on appropriate analysis of genetic similarity provided by Prof. Staffan Bensch, Lund University).
All variables, except fertilization success in controls, met the assumptions of normality and homogeneity of variances. We detected no significant variation in fertilization success among sperm competition replicates within a trial (ANOVA F31,58=2.45, p=0.10), and all further analyses are therefore conducted on the mean for the three replicates in a sperm competition trial.
We analysed co-variation between the difference in the proportion of offspring sired by each male in sperm competition and the difference in (i) genetic similarity between the female and each of the males, (ii) mass of the two males relative to the female (i.e. (mass male 1−female mass)−(mass male 2−female mass)), (iii) the proportion of viable sperm between males, (iv) initial sperm concentration between males, (v) hatching success in the controls, and (vi) fertilization success in the controls. We then investigated the relative importance of these effects in determining siring success of males in sperm competition using a general linear model (Proc GLM, SAS v. 9.1). However, since fertilization success in the controls could not be transformed to normality, we verified the results from the parametric analyses with non-parametric statistics using a Spearman's partial rank-order correlation analysis in SAS v. 9.1.
3. Results
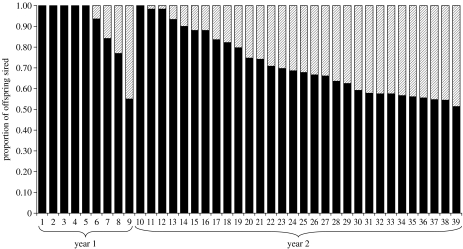
In sperm competition trials from the first year of this study, eight of the nine trials showed highly skewed paternity towards one of the two potential sires (figure 1). In five of the trials, 100% of the offspring were sired by one of the two competing male ejaculates. In only one trial, we detected a near 50 : 50 ratio in siring success (trial 9; figure 1), suggesting that sperm number by itself is insufficient for determining a male's success in sperm competition.
Figure 1.
The percentage of offspring sired by either male 1 (black) or male 2 (hatched) in 39 sperm competition trials over 2 years in the Australian Peron's tree frog L. peronii.
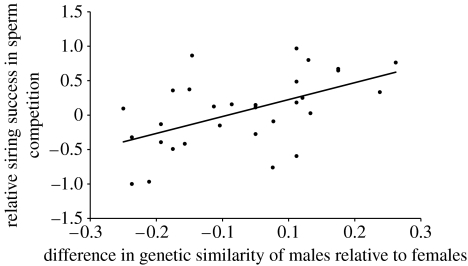
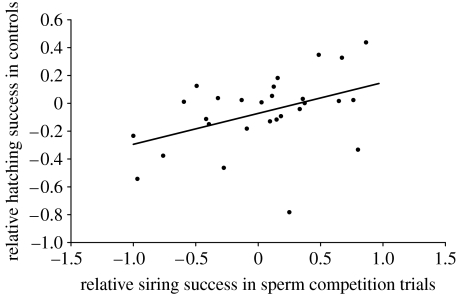
In sperm competition trials from the second year, we again detected highly skewed paternity in most trials (figure 1). Following backward elimination (p>0.25) of initial sperm concentration and proportion of viable sperm, we conducted a generalized linear model analysis, with the four remaining predictors that all demonstrated positive significant effects on male siring success (F4,25=11.6, p<0.0001, R2=0.65; table 1). The differences in (i) proportion of fertilized eggs in controls, (ii) body mass relative to female mass, (iii) hatching success in controls (which may affect the number of counted offspring sired by a male), and (iv) relative relatedness with the female, each explained 9, 10, 18 and 28%, respectively, of the variation in siring success (table 1; explained variance calculated by backward elimination by taking the differences in R2 values between models). Since the difference in fertilization success in controls could not be normalized, we re-examined our results in the most conservative way possible by calculating Spearman's partial rank-order correlations (Proc Corr, SAS) between the difference in siring success and each predictor variable, while holding remaining predictors constant. The only relationship that held up for this scrutiny was the relationship between difference in male relatedness with the female and siring success (figure 2), which remained significant also after Bonferroni correction of the α-value to 0.0125 (table 1). We also detected a significant relationship between difference in siring success in sperm competition trials and the hatching success of fertilized eggs in control Petri dishes (Pearson's correlation, r=0.44, p=0.018; figure 3).
Table 1.
Multiple regression analysis of predictors of the difference in siring success between sperm from two different males. (The test statistics are given to the left in the table including the regression coefficients for the predictors (β±s.e.), while the corresponding analysis of variance is given in the right. ΔRelatedness, difference in male relatedness to the female; Δhatching, difference between males in hatching success in the controls; Δmass, difference in male body size relative to the female and Δfertility, difference in male fertilization success in controls. The rs statistic represents Spearman's partial rank-order correlation with all other variables held constant. Italic values represent a statistically significant relationship after Bonferroni correction. Model F4,25=11.6, p=<0.0001, R2=0.65.)
| source | β±s.e. | t | p | d.f. | F | p | rs | p |
|---|---|---|---|---|---|---|---|---|
| Δrelatedness | 1.81±0.60 | 2.9 | 0.005 | 1 | 9.26 | 0.005 | 0.55 | 0.003 |
| Δhatching | 0.50±0.24 | 2.1 | 0.047 | 1 | 4.34 | 0.047 | 0.36 | 0.061 |
| Δmass | 0.09±0.04 | 2.0 | 0.052 | 1 | 2.04 | 0.052 | 0.27 | 0.175 |
| Δfertility | 0.70±0.28 | 2.5 | 0.020 | 1 | 2.48 | 0.020 | 0.31 | 0.112 |
Figure 2.
Relationship between the relative genetic similarity of males to the females mated and relative siring success of males in 30 sperm competition experiments.
Figure 3.
Relationship between the relative siring success of males in 30 sperm competition experiments and the relative hatching success of males in controls (Pearson's correlation, r=0.44, p=0.018).
Our hybridization experiment confirmed that the eggs of L. peronii were successfully fertilized in all the three trials by sperm from the sympatric congeneric species L. tyleri (mean±s.e., 55±5% of the eggs).
4. Discussion
Our study shows that fertilization success in L. peronii is often skewed towards one of the two competing males in sperm competition even when the number of sperm within ejaculates is standardized. Siring success was not related to the proportion of viable sperm within a male's ejaculate. Instead, we found that males more genetically similar to a female fertilized a greater proportion of her eggs in sperm competition trials and in controls, suggesting some form of sperm selection at the gametic level. These results contrast sharply with most other studies that have found a negative relationship between siring success and genetic similarity (e.g. Olsson et al. 1996; Penn & Potts 1998, 1999; Tregenza & Wedell 2000; Landry et al. 2001; Kraaijeveld-Smit et al. 2002). However, the majority of these studies have typically been based on laboratory or inbred populations. Studies from natural populations are limited in number, although some have reported greater siring success between individuals with intermediate levels of genetic similarity (optimal outbreeding; Bateson 1982, 1983; Cohen & Dearborn 2004; Thünken et al. 2007), while other studies have found no effect at all (e.g. Stockley 1997). To our knowledge, this study is the first to show post-copulatory selection of sperm from genetically more similar individuals within a population.
Previous work on lizards demonstrate that neutral markers such as DNA fingerprinting and microsatellite markers have the capacity to, in some species, identify relevant genetic variation, or may be linked with key loci that are associated with partner discrimination at a post-copulatory level (Olsson et al. 1994, 1996). Reproductive proteins involved in these processes have the capacity to evolve rapidly not only in contexts of hybridization avoidance and speciation (Swanson & Vacquier 2002), but may also evolve into within-species recognition systems between males and females of different genotypes. Examples of within-species recognition systems include the highly variable S-locus genes, SCR and SRK, in plants (Schopfer et al. 1999) and ZP genes in mammals (Swanson & Vacquier 2002), which may result in male×female interaction effects on fertilization probabilities and offspring viability (Clark et al. 1999). None of these processes have, to the best of our knowledge, been studied in amphibians, in spite of them having served as models in fertilization biology for decades (e.g. Gomez & Cabada 1994 and references therein).
From a population genetics perspective, the overall level of genetic similarity between individuals within our sample was very low (mean percentage of alleles shared between males and females±s.d., 21±9%). Thus, males and females that were deemed genetically similar in this study still shared only a small proportion of alleles and, therefore, mating between such individuals may not result in inbreeding depression. Nevertheless, our control fertilizations showed that males more successful in sperm competition with particular females were also more successful at fertilizing eggs in control fertilization trials with eggs from the same females, and had a higher hatching success. Thus, siring success is clearly linked to some unidentified sperm–egg interaction that increases both the probability of fertilization and subsequent hatching success of tadpoles. This can clearly not be the result of maternal effects only (since sperm from the males more related to the females does better when fertilizing eggs in controls than the less related males), and also not be the result of more or ‘better’ spermatozoa from target males, since neither of these parameters influenced a male's siring success in sperm competition or fertilization success in controls. However, other traits such as sperm motility and velocity have been shown to be important in determining fertilization success in a number of external fertilizing species and were not measured in this study (e.g. Gage et al. 2004; Casselman et al. 2006). Additionally, significant intra-population variation in sperm traits such as motility has been reported in other amphibians (Hettyey & Roberts 2006). Thus, we cannot rule out that sperm motility and velocity can account for some of the unexplained variation in fertilization success detected among males. However, if sperm motility or velocity were a main factor driving fertilization success, we would have expected to see variation in siring success among males in the sperm competition trials (with the fastest sperm ‘winning out’) but not in the control fertilizations (as there is no competition and perhaps sufficient time for fertilization to occur regardless of how fast sperm swim). However, our results showed that siring success in the sperm competition trials was consistent with the hatching success in control fertilizations, with males doing poorly in controls also doing poorly in sperm competition. Thus, sperm velocity or motility is unlikely to be a significant factor determining fertilization success in our experiments.
The observed pattern may be driven by selection processes such as hybridization avoidance. Litoria tyleri co-occurs with L. peronii at our study location and has an overlapping breeding season with L. peronii. The three laboratory crosses between male L. tyleri and female L. peronii resulted in viable offspring (although their subsequent survival in the wild was never assessed). Thus, if there is a risk of hybridization between these two species during spawning, and hybrid offspring have lower fitness, selection should favour fertilization mechanisms that biased the paternity towards genetically more similar males.
Though ‘sperm selection’ by the female's eggs appears to be the most likely explanation for our results, the possibility that some male effect is driving the non-random paternity with respect to genetic similarity cannot be ruled out. The strong correlation between a male's siring success in both sperm competition and fertilization success in controls indicates that some form of genetic compatibility mechanism may be acting in this species.
In conclusion, given the strong relationship between genetic similarity and siring success, we suggest that our results are best explained by an undetected gametic recognition system that may prevent hybridization between closely related sympatric species than by straightforward sperm competition.
Acknowledgments
All work was carried out in accordance with National Parks and Wildlife Services permit S11186 and the Wollongong University animal ethics permits AE04/03-05.
We thank Ken Griffiths, Carlos Reyes, Glen Murray and Adam Moore for their help with field and laboratory work, and the Fahey family for access to breeding ponds at Darkes forest. We also thank Prof. Staffan Bensch for advice and comments on appropriate analysis of genetic similarity and four anonymous referees for their comments on previous versions of this manuscript. This work was supported by Australian Research Council Discovery Grants to M.O. and E.W. (grant no. DP0559867), and T.U. (grant no. DP0664403) and the Wenner-Gren Foundation (T.U.).
References
- Abt G, Reyer H.U. Mate choice and fitness in a hybrid frog —Rana esculenta females prefer Rana lessonae males over their own. Behav. Ecol. Sociobiol. 1993;32:221–228. doi:10.1007/BF00166511 [Google Scholar]
- Arnold M.L. Oxford University Press; Oxford, UK: 1997. Natural hybridization and evolution. [Google Scholar]
- Bateson P. Preferences for cousins in Japanese Quail. Nature. 1982;295:236–237. doi:10.1038/295236a0 [Google Scholar]
- Bateson P. Cambridge University Press; Cambridge, UK: 1983. Mate choice. [Google Scholar]
- Berger L. Systematics and hybridization in the Rana esculenta complex. In: Taylor D.H, Guttmann S.I, editors. The reproductive biology of amphibians. Plenum Press; New York, NY: 1977. pp. 367–388. [Google Scholar]
- Berger L, Rybacki M, Hotz H. Artificial fertilization of water frogs. Amphib.-reptil. 1994;15:408–413. doi:10.1163/156853894X00452 [Google Scholar]
- Bernal X.E, Stanley Rand A, Ryan M.J. Sex differences in response to nonconspecific advertisement calls: receiver permissiveness in male and female tungara frogs. Anim. Behav. 2007;73:955–964. doi:10.1016/j.anbehav.2006.10.018 [Google Scholar]
- Birkhead T.R. Cryptic female choice: criteria for establishing female sperm choice. Evolution. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. doi:10.2307/2411251 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Pizzari T. Postcopulatory sexual selection. Nat. Rev. Genet. 2002;3:262–273. doi: 10.1038/nrg774. doi:10.1038/nrg774 [DOI] [PubMed] [Google Scholar]
- Casselman S.J, Schulte-Hostedde A.I, Montgomerie R. Sperm quality influences male fertilization success in walleye (Sander vitreus) Can. J. Fish. Aquat. Sci. 2006;63:2119–2125. doi:10.1139/F06-108 [Google Scholar]
- Clark A.G, Begun D.J, Prout T. Female×male interactions in Drosophila sperm competition. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. doi:10.1126/science.283.5399.217 [DOI] [PubMed] [Google Scholar]
- Cogger H. 6th edn. Reed New Holland; Sydney, Australia: 2000. Reptiles and amphibians of Australia. [Google Scholar]
- Cohen L.B, Dearborn D.C. Great frigatebirds, Fregata minor, choose mates that are genetically similar. Anim. Behav. 2004;68:1229–1236. doi:10.1016/j.anbehav.2003.12.021 [Google Scholar]
- Dolgin E.S, Charlesworth B, Baird S.E, Cutter A.D. Inbreeding and outbreeding depression in Caenorhabditis nematodes. Evolution. 2007;61:1339–1352. doi: 10.1111/j.1558-5646.2007.00118.x. doi:10.1111/j.1558-5646.2007.00118.x [DOI] [PubMed] [Google Scholar]
- Engeler B, Reyer H.U. Choosy females and indiscriminate males: mate choice in mixed populations of sexual and hybridogenetic water frogs (Rana lessonae, Rana esculenta) Behav. Ecol. 2001;12:600–606. doi:10.1093/beheco/12.5.600 [Google Scholar]
- Frankham R. Conservation genetics. Annu. Rev. Genet. 1995;29:305–327. doi: 10.1146/annurev.ge.29.120195.001513. doi:10.1146/annurev.ge.29.120195.001513 [DOI] [PubMed] [Google Scholar]
- Frankham R. Genetics and extinction. Biol. Conserv. 2005;126:131–140. doi:10.1016/j.biocon.2005.05.002 [Google Scholar]
- Gage M.J.G, Macfarlane C.P, Yeates S, Ward R.G, Searle J.B, Parker G.A. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 2004;14:44–47. [PubMed] [Google Scholar]
- Gomez M.I, Cabada M.O. Amphibian cross-fertilization and polyspermy. J. Exp. Zool. 1994;269:560–565. doi: 10.1002/jez.1402690609. doi:10.1002/jez.1402690609 [DOI] [PubMed] [Google Scholar]
- Griffiths K. University of New South Wales Press; Sydney, Australia: 2006. Frogs and reptiles of the Sydney region. [Google Scholar]
- Hettyey A, Roberts J.D. Sperm traits of the quacking frog, Crinia georgiana: intra- and interpopulation variation in a species with a high risk of sperm competition. Behav. Ecol. Sociobiol. 2006;59:389–396. doi:10.1007/s00265-005-0062-3 [Google Scholar]
- Hoskin C.J, Higgie M, McDonald K.R, Moritz C. Reinforcement drives rapid allopatric speciation. Nature. 2005;437:1353–1356. doi: 10.1038/nature04004. doi:10.1038/nature04004 [DOI] [PubMed] [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin's finches. Evolution. 2002;56:1229–1239. doi: 10.1111/j.0014-3820.2002.tb01434.x. doi:10.1111/j.0014-3820.2002.tb01434.x [DOI] [PubMed] [Google Scholar]
- Kokko H, Ots I. When not to avoid inbreeding. Evolution. 2006;60:467–475. doi:10.1111/j.0014-3820.2006.tb01128.x [PubMed] [Google Scholar]
- Kraaijeveld-Smit F.J.L, Ward S.J, Temple-Smith P.D, Paetkau D. Factors influencing paternity success in Antechinus agilis: last-male sperm precedence, timing of mating and genetic compatibility. J. Evol. Biol. 2002;15:100–107. doi:10.1046/j.1420-9101.2002.00367.x [Google Scholar]
- Landry C, Garant D, Duchesne P, Bernatchez L. ‘Good genes as heterozygosity’: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar) Proc. R. Soc. B. 2001;268:1279–1285. doi: 10.1098/rspb.2001.1659. doi:10.1098/rspb.2001.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBas N.R. Mate choice, genetic incompatibility, and outbreeding in the ornate dragon lizard, Ctenophorus ornatus. Evolution. 2002;56:371–377. doi: 10.1111/j.0014-3820.2002.tb01347.x. doi:10.1111/j.0014-3820.2002.tb01347.x [DOI] [PubMed] [Google Scholar]
- Madsen T, Shine R, Olsson M, Wittzell H. Conservation biology—restoration of an inbred adder population. Nature. 1999;402:34–35. doi:10.1038/46941 [Google Scholar]
- Martin P.A, Dziuk P.J. Assessment of relative fertility of males (cockerels and boars) by competitive mating. J. Reprod. Fert. 1977;49:323–329. doi: 10.1530/jrf.0.0490323. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Fertility, mating behaviour and sexual selection in Drosophila subobscura. J. Genet. 1956;54:261–279. doi: 10.1007/BF02715886. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Attitudes to animal behavior. In: Singh R.S, Krimbas C.B, editors. Evolutionary genetics: from molecules to morphology. Cambridge University Press; Cambridge, UK: 2000. pp. 628–640. [Google Scholar]
- Neff B.D. Stabilizing selection on genomic divergence in a wild fish population. Proc. Natl Acad. Sci. USA. 2004;101:2381–2385. doi: 10.1073/pnas.0307522100. doi:10.1073/pnas.0307522100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Olsson M, Gullberg A, Tegelstrom H, Madsen T, Shine R. Can female adders multiply? Nature. 1994;369:528. doi:10.1038/369528b0 [Google Scholar]
- Olsson M, Shine R, Madsen T, Gullberg A, Tegelstrom H. Sperm selection by females. Nature. 1996;383:585. doi:10.1038/383585a0 [Google Scholar]
- Palumbi S.R. All males are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc. Natl Acad. Sci. USA. 1999;96:12 632–12 637. doi: 10.1073/pnas.96.22.12632. doi:10.1073/pnas.96.22.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer K, Taborskyi M. Outbreeding depression, but no inbreeding depression in haplodiploid ambrosia beetles with regular sibling mating. Evolution. 2005;59:317–323. doi:10.1111/j.0014-3820.2005.tb00992.x [PubMed] [Google Scholar]
- Penn D.J, Potts W.K. MHC-disassortative mating preferences reversed by cross-fostering. Proc. R. Soc. B. 1998;265:1299–1306. doi: 10.1098/rspb.1998.0433. doi:10.1098/rspb.1998.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn D.J, Potts W.K. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 1999;153:145–164. doi: 10.1086/303166. doi:10.1086/303166 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Inbreeding depression: tests of the overdominance and partial dominance hypotheses. Evolution. 2002;56:768–775. doi: 10.1111/j.0014-3820.2002.tb01387.x. doi:10.1111/j.0014-3820.2002.tb01387.x [DOI] [PubMed] [Google Scholar]
- Schjørring S, Jäger I. Incestuous mate preference by a simultaneous hermaphrodite with strong inbreeding depression. Evolution. 2007;61:423–430. doi: 10.1111/j.1558-5646.2007.00028.x. doi:10.1111/j.1558-5646.2007.00028.x [DOI] [PubMed] [Google Scholar]
- Schopfer C.R, Nasrallah M.E, Nasrallah J.B. The male determinant of self-incompatibility in Brassica. Science. 1999;286:1697–1700. doi: 10.1126/science.286.5445.1697. doi:10.1126/science.286.5445.1697 [DOI] [PubMed] [Google Scholar]
- Sherman C.D.H, Olsson M. Polymorphic microsatellite loci in the Australian tree frog, Litoria peronii. Conserv. Genet. 2007;8:999–1001. doi:10.1007/s10592-006-9218-6 [Google Scholar]
- Sherman, C. D. H., Wapstra, E., Uller, T. & Olsson, M. In press. Male and female effects on fertilization success and offspring viability in the Peron's tree frog, Litoria peronii. Austral. Ecol. (doi:10.1111/j.1442-9993.2007.01823.x)
- Simmons L.W. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 2005;36:125–146. doi:10.1146/annurev.ecolsys.36.102403.112501 [Google Scholar]
- Stockley P. No evidence of sperm selection by female common shrews. Proc. R. Soc. B. 1997;264:1497–1500. doi: 10.1098/rspb.1997.0207. doi:10.1098/rspb.1997.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson W.J, Vacquier V.D. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 2002;3:137–144. doi: 10.1038/nrg733. doi:10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- Szymura J.M. Analysis of hybrid zones with Bombina. In: Harrison R.G, editor. Hybrid zones and the evolutionary process. Oxford University Press; Oxford, UK: 1993. pp. 261–289. [Google Scholar]
- Thünken T, Bakker T.C.M, Baldauf S.A, Kullmann H. Active inbreeding in a cichlid fish and its adaptive significance. Curr. Biol. 2007;17:225–229. doi: 10.1016/j.cub.2006.11.053. doi:10.1016/j.cub.2006.11.053 [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Tymchuk W.E, Sundstrom L.F, Devlin R.H. Growth and survival trade-offs and outbreeding depression in rainbow trout (Oncorhynchus mykiss) Evolution. 2007;61:1225–1237. doi: 10.1111/j.1558-5646.2007.00102.x. doi:10.1111/j.1558-5646.2007.00102.x [DOI] [PubMed] [Google Scholar]
- Vörös J, Alcobendas M, Martinez-Solano I, Garcia-Paris M. Evolution of Bombina bombina and Bombina variegata (Anura: Discoglossidae) in the Carpathian Basin: a history of repeated mt-DNA introgression across species. Mol. Phylogenet. Evol. 2006;38:705–718. doi: 10.1016/j.ympev.2005.08.010. doi:10.1016/j.ympev.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Waits L.P, Luikart G, Taberlet P. Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol. Ecol. 2001;10:249–256. doi: 10.1046/j.1365-294x.2001.01185.x. doi:10.1046/j.1365-294X.2001.01185.x [DOI] [PubMed] [Google Scholar]
- Waser N.M, Price M.V, Shaw R.G. Outbreeding depression varies among cohorts of Ipomopsis aggregata planted in nature. Evolution. 2000;54:485–491. doi: 10.1111/j.0014-3820.2000.tb00051.x. doi:10.1111/j.0014-3820.2000.tb00051.x [DOI] [PubMed] [Google Scholar]
- Wright, S. 1933 The roles of mutation, inbreeding, crossbreeding and selection in evolution. In Proc. VIth Int. Congress on Genetics, vol. 1 (ed. D. F. Jones), pp. 356–366. New York, NY: Brooklyn Botanic Garden.