Abstract
Certain insect species are known to relocate nest or food sites using landmarks, but the generality of this capability among insects, and whether insect place memory can be used in novel task settings, is not known. We tested the ability of crickets to use surrounding visual cues to relocate an invisible target in an analogue of the Morris water maze, a standard paradigm for spatial memory tests on rodents. Adult female Gryllus bimaculatus were released into an arena with a floor heated to an aversive temperature, with one hidden cool spot. Over 10 trials, the time taken to find the cool spot decreased significantly. The best performance was obtained when a natural scene was provided on the arena walls. Animals can relocate the position from novel starting points. When the scene is rotated, they preferentially approach the fictive target position corresponding to the rotation. We note that this navigational capability does not necessarily imply the animal has an internal spatial representation.
Keywords: insect learning, visual navigation, place memory, cricket (Gryllus bimaculatus), homing algorithms
1. Introduction
Since Tinbergen & Kruy's (1938) experiments demonstrated that a wasp will search for its nest in the position indicated by surrounding visual features, a great deal of evidence about the visual navigation capabilities of insects has accumulated. Studies have focused on nest-based foraging, particularly in wasps, ants and bees, with a number of impressive results; although as yet there is not an established consensus on the mechanism by which visual cues are stored, recalled and used to relocate the nest or the food source. It also remains an open question just how general visual place memory across insect species is, and whether insects can use this ability in novel task settings.
Mizunami et al. (1993, 1998b) devised a test for place memory in the cockroach Periplaneta americana based on the classic water maze paradigm used for rodents (Morris 1981). The animal is placed in an unpleasant environment (for rats, a pool of water; for cockroaches, a heated metal arena) and is thus motivated to move until it locates a safe position (an underwater platform or a cool spot, respectively). Several lines of evidence are used to argue that the animal locates the (invisible) safe position using the surrounding visual landmarks (outside the pool or on the sides of the arena), i.e. that it has formed a ‘place memory’. It can relocate the position from novel starting points in subsequent trials, and will search preferentially in that position on trials when the platform or cool spot is not present. It is less successful in learning the task when no visual cues are provided. Moreover, when the visual cues are rotated, the animal will search in the vicinity of the ‘fictive’ target indicated by those cues.
The water maze paradigm has been used extensively as an assay for the spatial memory capabilities of rodents, but almost 10 years have passed without any reported follow-up studies in insects using the Mizunami et al. approach (which has been dubbed the Tennessee Williams paradigm). To the authors' knowledge, only Scotto-Lomassese et al. report that they attempted to test the ability of the house cricket Acheta domesticus on this paradigm but found that ‘the first motivation of crickets was to escape from the closed arena’ (Scotto-Lomassese et al. 2003). It should also be admitted that the data in the original Mizunami et al. study are suggestive rather than conclusive: due to substantial variability in behaviour, no statistical significance of the apparent improvement in locating the target over 10 trials is reported, and searching in the fictive location after cue rotation is reported only for two individuals. However, the main point of their report is the demonstration that ablation of the mushroom body (MB) neuropils in the cockroach significantly affects the performance on this task but does not change the performance when the target itself is visible.
In this paper, we report on the behaviour of the cricket Gryllus bimaculatus in the Tennessee Williams paradigm. Cricket visual navigation capabilities have not been extensively studied to date. Crickets have polarized light vision (Brunner & Labhart 1987) and appear to be able to use this in maintaining a course (Weber 1990). They have been shown to learn a compass direction towards a shoreline after single trial of evasive swimming (Beugnon 1986). They may also use a polarization compass in path integration, e.g. to relocate their burrow (Beugnon & Campan 1989). However, in the latter study, Beugnon & Campan found little evidence that Gryllus campestris could home to its burrow using other visual cues (except at distances less than 20 cm away when the burrow itself is visible). Other cricket species that live on habitat borders appear to use visual cues to return to the border (Honegger & Campan 1989). Recently, it has been shown that juvenile raspy crickets Gryllacrididae will use spatial cues in a simple two-choice maze to return to their burrow (Hale & Bailey 2004).
Here we demonstrate that crickets can learn to use visual surroundings to locate an invisible target position in a novel task situation. We discuss insights derived from these experiments regarding the possible mechanisms of visual homing in insects.
2. Material and methods
(a) Animals
Adult female G. bimaculatus crickets were isolated after their final moult and maintained individually in small plastic cages under a 12 L : 12 D cycle. The animals were kept at 21±1°C and were fed water and dog food.
(b) General procedure
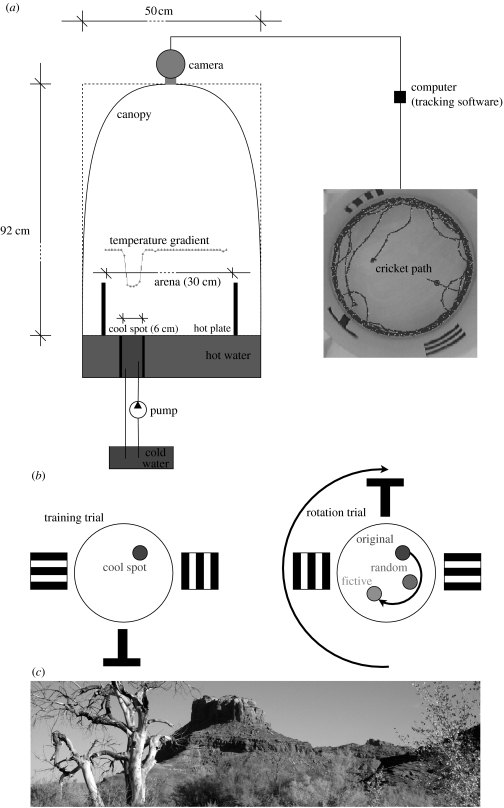
In the ‘Tennessee Williams’ paradigm, the cricket is placed in a circular arena with a metal floor on the top of a water tank. The water is heated to approximately 50°C which in turn heats the metal floor surface of the arena, and to some extent, the surrounding the metal wall. A single circular cool spot on the arena floor is created by continuously circulating cool water through a separate, insulated compartment of the water tank (figure 1a). This target is visually and texturally indistinguishable from the surrounding area.
Figure 1.
Experimental set-up. (a) The arena was illuminated by two desk lamps. The temperature gradient was measured at the surface (min∼25° and max∼50°). (b) For the rotation trials, the wall of the arena is rotated by 180°, changing the position of the visual cues, and creating a fictive target location relative to those cues. (c) This natural scene wallpaper is wrapped around the inside of the arena wall.
The cricket is placed down at a random location in the arena and allowed to move about freely. The trial ends if the cricket finds the cool spot and remains there for 30 s or if the trial has lasted 5 min without the cricket finding the target (to prevent heat-shock). If the cricket has not located the cool spot during a trial it is placed on the cool spot (under a glass) for 30 s at the end of the trial. The crickets are rested for 2 min between trials in an opaque beaker. Between trials, the arena is wiped clean to remove any olfactory cues left by the animal.
(c) Experiments
Our first experiment aimed to replicate, using crickets, the tests performed on cockroaches by Mizunami et al. (1998b). The arena diameter was 30 cm and the target cool spot diameter approximately 6 cm. Animals were tested under three conditions: with a visible target—a small metal plate with a different surface colour placed over the cool spot (n=6); with an invisible target and artificial visual cues on the walls—a black T shape, horizontal and vertical stripes (n=12) as shown in figure 1a; and with no visible target or cues (n=9). In all conditions, the arena was covered by a white canopy to reduce the possibility of the animals using external visual cues, such as laboratory furniture or lighting. However, as described in the results section, this probably did not provide a completely uniform visual field. In each condition, each cricket was tested on 10 successive trials.
In the second experiment, we increased the arena diameter to 40 cm, thus approximately doubling the ratio of arena to target area, reducing the likelihood that the cricket could find the cool spot using some random search strategy. The target was always invisible, and we used four visual cue conditions. The first was three simple black and white shapes on the arena wall as in experiment 1. The second was a natural scene, as shown in figure 1c, wrapped as a panorama on the arena wall. Our main motivation for including this was that one of the known algorithms for visual homing (see §4) functions better with natural scene statistics than with highly simplified cues. The third was a ‘no cues’ condition with blank walls. For each of these conditions, we used a dark canopy to try to further reduce any additional visual stimuli. Finally, as a control experiment, trials were carried out in a completely dark arena thus removing all visual cues. As for the first experiment, there were 10 learning trials for each condition. After the 10th trial, we also tested the crickets with the arena wall, and thus the visual cues for the first two conditions, rotated (cf. figure 1b). The floor was evenly heated. If the animal is using the visual cues to determine the target location, this manipulation should create a fictive target location, relative to the cues. If it is not using these cues, the fictive position should be no more attractive than the original target position or any other random point in the arena at a similar distance from the walls.
(d) Data analysis
An overhead web camera (Logitech) is used to record the behaviour of the animals directly on the computer at 5 frames s−1. For the dark control experiments, the infrared filter on the camera was removed. Cricket positions are extracted from the video recordings with customized tracking software developed by Rosano & Webb (2007). The data are then further analysed with scripts written in or provided by Matlab. We extract the following measures from the captured data: time to reach certain areas in the arena, path length, velocity and time spent in vicinity of the wall. Non-parametric pairwise comparisons are made to establish significant differences between conditions and trials.
3. Results
(a) Experiment 1
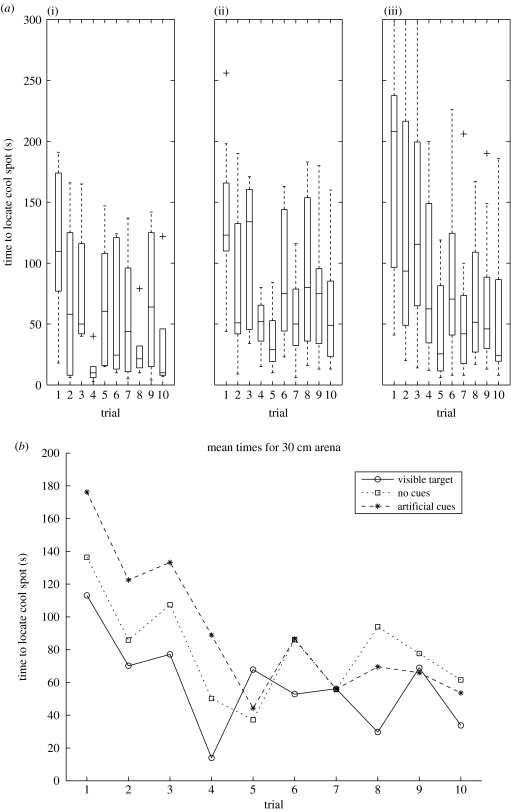
We compared learning performance with a visible target, with no cues, and with artificial cues on the walls in a 30 cm arena. Boxplots showing the time taken to locate the cool spot over the 10 trials are shown in figure 2a, and the mean times for the three conditions plotted against trial numbers in figure 2b.
Figure 2.
Experiment 1 results. Time taken to find the cool spot over 10 trials in 30 cm arena (a) with (i) a visible target (n=6), (ii) no cues (n=9) or (iii) visual cues on the arena wall (n=12); (b) a comparison of mean times for the three conditions. Learning occurs in all three conditions.
It is apparent from the graphs that the time taken to locate the target decreases over the first five trials. Comparing trials 1 and 10 showed a significant improvement in all conditions (Wilcoxon signed-rank test: no cues, p<0.005; cues, p<0.001; visible target, p=0.06). While this suggests that crickets are indeed learning to locate the cool spot, the similarity of behaviour with cues and no cues suggests either that the ‘no cues’ condition does in fact contain some cues, such as visual cues above the arena walls caused by shadows on the canopy from the structure supporting the camera, or that the insect is using some strategy other than visual memory to locate the target more quickly on successive trials.
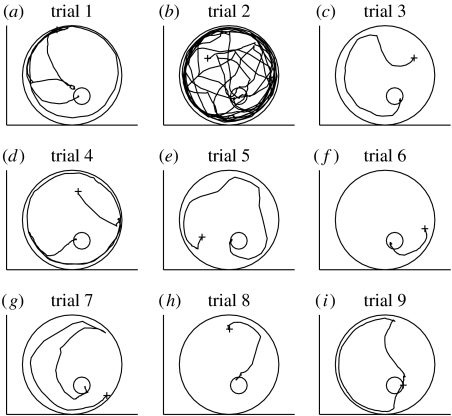
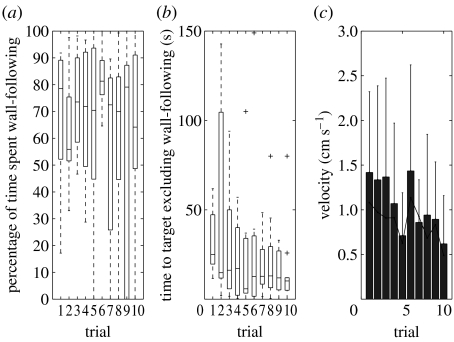
A closer examination of the tracks provided some insight (see figure 3). It is worth noting that the cricket approaches the target position from a variety of directions, thus it is not simply acquiring some stereotyped motor response. Most crickets on being introduced to the arena showed a strong tendency to run towards and along the walls, presumably trying to escape the arena. These wall-following bouts would often recur in later trials, even after the animal had several times previously moved directly to the target (and even when the target was visible). This added much variability to the time taken to locate the target. In figure 4, we plot the percentage of time spent wall-following (defined as a track falling within 4 cm of the walls) for the trials with artificial visual cues, and show the residual durations of the tracks excluding the wall-following. The learning across trials is still evident and it does not appear to be explained simply by the animal reducing the time spent wall-following, i.e. wall-following explains some of the pattern but not the overall trend towards faster times. We also note that the faster time to locate the target is not simply due to faster movement by the animal, as the average velocity tended to decrease over the 10 trials (figure 4c).
Figure 3.
Cricket paths. Paths from (a–i) nine trials (trials 1–9) for one cricket with artificial visual cues. Cross, start of the path; small circle, the hidden target location.
Figure 4.
Wall-following. For the visual cues condition, (a) the percentage of time spent wall-following and (b) the time to locate target excluding time spent wall-following. (c) Mean and standard deviation of the crickets' velocity against trial numbers (the black line represents the median).
There was also some evidence that crickets found the visual cues themselves attractive (initial use of a solid black square target had to be abandoned as this proved strongly attractive). However, on at least some trials with visual cues, the cricket would stop at some point in the arena, fixate each of the visual cues and then move quite directly to the target location, suggesting that visual memory of the relation of distant cues to the target could be employed. Finally, on other trials, both with and without cues, it was observed that some crickets would circle the walls but make repeated deviations away from them, which was also a moderately successful strategy for encountering the target. In a 30 cm arena, the chance of finding the target once away from the walls is quite high. Perhaps the task is not sufficiently difficult to fully reveal the use of visual memory?
(b) Experiment 2
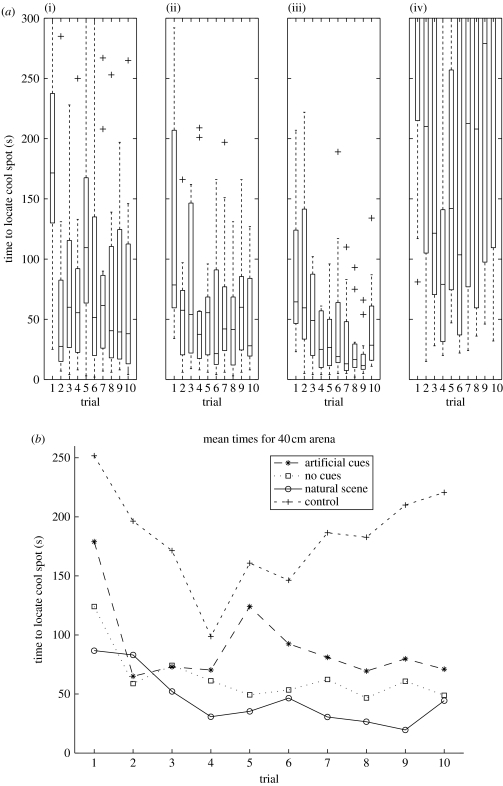
Using an increased arena diameter of 40 cm, we compared the learning performance with artificial cues, natural cues, no cues and in the dark. The animals show improved performance when comparing trials 1 and 10 in all conditions except for the dark (Wilcoxon signed-rank test: artificial cues, p<0.01; no cues, p<0.01; natural cues, p=0.06; in the dark, p>0.4), see boxplots in figure 5a. For the artificial cues, there seems to be a sharp improvement on the second trial but no further improvement; performance from trials 4–10 is slightly worse than it was in the 30 cm arena (comparing the average times across these trials, 84 versus 61 s, Wilcoxon rank-sum test: p<0.05). Comparing the means for the three conditions in the 40 cm arena (figure 5b) it is clear that the improvement is the greatest for the ‘natural cues’ condition, but also, perhaps surprisingly, that the artificial cues on the walls produce worse results than ‘no cues’ (comparing averages across trials 4–10 using Wilcoxon rank-sum test: natural versus artificial cues, 33 versus 84 s, p<0.001; natural versus no cues, 33 versus 55 s, p<0.05; artificial versus no cues, 84 versus 55 s, p<0.05). All the three conditions are significantly different from the control dark condition (average 172 s).
Figure 5.
Experiment 2 results. Time taken to find the hidden cool spot over 10 trials in an 40 cm arena (a) with (i) artificial cues on the arena wall (n=12), (ii) no cues (n=12), (iii) a natural scene on the arena wall (n=12) and (iv) control experiment in the dark (n=12); (b) a comparison of mean times for all conditions.
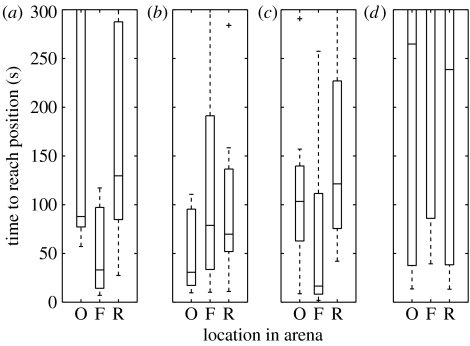
Figure 6 shows the results for the rotation tests with artificial, natural, no cues on the arena and in the dark. The time from the beginning of the trial until the cricket's track crosses the original location of the cool spot, the fictive location indicated by the cues or panorama, or a random location of an equal size and equal distance from the walls is shown in the boxplots. Each location is defined as a 2 cm radius circle. For natural cues, the time to reach the fictive location is significantly faster than the time to reach the original location or a random position (sign test, original versus fictive, p=0.0386). For artificial cues, the same pattern was observed but the results were of marginal significance (original versus fictive, p=0.11). For ‘no cues’, crickets seem to reach the original location more quickly suggesting that they may be able to use other information (that has not been rotated) than the wall cues for locating the hidden target location. For the control condition, crickets show no preference and no attraction to any of the test locations.
Figure 6.
Results on rotation trials. For (a) artificial cues and (c) the natural scene, the fictive position of the cool spot indicated by the rotated cues or panorama is approached more quickly than the original location, or a random position. (b) For ‘no cues on arena wall’, the original position is approached more quickly than the fictive or random locations. (d) In the control experiment, no spot, original, fictive or random, is approached significantly faster. O, original cool spot; F, fictive; R, random.
4. Discussion
The results presented here demonstrate that the cricket is capable of using surrounding visual cues to relocate a hidden target position, in an arbitrary task setting. This is suggested by the observed performance improvement over trials, and the ability to relocate the cool spot from different starting positions, but is most strongly supported by the results of the rotation trials. In these trials, only the visual cues on the arena walls were changed, controlling for all other possible sensory cues that might potentially have contributed to the observed learning, and a corresponding change in the cricket's preferred location was observed. Hence, memory of the surrounding visual cues is sufficient for localization in this task.
The unexpected observation of significantly improved performance in the ‘no cues’ conditions initially suggests that visual cues might be sufficient but are not necessary. However, floor, wall and canopy were coloured differently and thus the animals may have been able to detect edges which—even if uniform in rotation—could be used to determine the distance from the walls at which to search for the target. Furthermore, the illumination was not uniform (see electronic supplementary material) and thus may also have provided subtle but sufficient rotational information. When all these residual cues were eliminated, the animals no longer showed a significant improvement in relocalization over the 10 trials. This suggests that visual input is in fact necessary for the task in this paradigm, although it is likely that under more natural conditions crickets are able to use other sensory cues to aid localization.
Performances of individual crickets varied greatly (see electronic supplementary material). Some crickets learned quickly and were able to relocate the cool spot consistently over all the remaining trials. Other crickets took longer to learn the location of the cool spot, or after apparently learning, would still sometimes exhibit bouts of wall-following behaviour (possibly exploratory or escape behaviour) lasting tens to hundreds of seconds. However, as we have shown in figure 4, if we exclude these bouts of wall-following from the total times taken to complete the trial, most animals are able to relocate the cool spot quite quickly from the fifth trial onward (median times approx. 15 s).
In Mizunami et al.'s study of this task in cockroaches, they found that an ablation of the MB neuropils significantly affects performance with the surrounding visual cues, but does not change performance when the target itself is visible. A large body of evidence supports a role for learning and memory of the mushroom bodies (e.g. summarized in Heisenberg 2003). The Tennessee Williams paradigm may help in elucidating the strategies of the visual homing behaviour of insects and in determining the involvement of the MB in visual homing. This paradigm may allow for the unique possibility to investigate the neural basis of visual homing in insects by recording from neurons in freely moving animals (e.g. Mizunami et al. 1998a).
As yet, there is no established consensus on the mechanism by which, during nest-based foraging, visual cues are stored, recalled and used to relocate the target. A number of algorithms have been proposed (e.g. Cartwright & Collett 1983; see also review in Franz & Mallot 2000) and demonstrated to work in simulation and on robot platforms. One simple solution, suggested by Zeil et al. (2003), is that an image similarity measure, such as the root mean square pixel difference between a panoramic image of the current position and a reference image (a snapshot taken at the goal location) can be used, in combination with a simple gradient descent algorithm, to return to the goal location. This mechanism has also been demonstrated to work in robotic implementations (Zampoglou et al. 2006) and is consistent with the improved behaviour found in our experiments for a complex natural scene compared with simple landmarks, as the difference gradient is more likely to be smooth for more complex scenes (Szenher 2005). It is important to note that the behaviour can thus potentially be explained without assuming the animal is storing a map-like representation of environmental space. This conclusion equally applies to rats in the Morris water maze.
Acknowledgments
We thank Hugh Cameron, Robert McGregor, Hugo Rosano and Sean Mangan for their technical support and assistance. This work was supported by the European Commission under grant FP6-2003-IST2-004690.
Supplementary Material
Additional figures and explanations
References
- Beugnon G. Learned orientation in landward swimming in the cricket Pteronemobius lineolatus. Behav. Process. 1986;12:215–226. doi: 10.1016/0376-6357(86)90037-9. doi:10.1016/0376-6357(86)90037-9 [DOI] [PubMed] [Google Scholar]
- Beugnon G, Campan R. Homing in the field cricket, Gryllus campestris. J. Insect Behav. 1989;2:187–198. doi:10.1007/BF01053291 [Google Scholar]
- Brunner D, Labhart T. Behavioural evidence for polarization vision in crickets. Physiol. Entomol. 1987;12:1–10. [Google Scholar]
- Cartwright B, Collett T. Landmark learning in bees: experiments and models. J. Comp. Physiol. A. 1983;151:521–543. doi:10.1007/BF00605469 [Google Scholar]
- Franz M, Mallot H. Biomimetic robot navigation. Robot. Auton. Syst. 2000;30:133–153. doi:10.1016/S0921-8890(99)00069-X [Google Scholar]
- Hale R, Bailey W. Homing behaviour of juvenile Australian raspy crickets (Orthoptera: Gryllacrididae) Physiol. Entomol. 2004;29:426–435. doi:10.1111/j.0307-6962.2004.00412.x [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nature. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Honegger, H.-W. & Campan, R. 1989 Vision and visually guided behavior. In Cricket behavior and neurobiology (eds F. Huber, T. E. Moore & W. Loher), pp. 147–177. Ithaca, NY: Cornell University Press.
- Mizunami M, Weibrecht J, Strausfeld N. A new role for the insect mushroom bodies: place memory and motor control. In: Beer R, editor. Biological neural networks in invertebrate neuroethology and robotics. Academic Press; Cambridge, UK: 1993. pp. 199–225. [Google Scholar]
- Mizunami M, Okada R, Li Y, Strausfeld N. Mushroom bodies of the cockroach: activity and identities of neurons recorded in freely moving animals. J. Comp. Neurol. 1998a;402:501–519. doi:10.1002/(SICI)1096-9861(19981228)402:4<501::AID-CNE5>3.0.CO;2-M [PubMed] [Google Scholar]
- Mizunami M, Weibrecht J, Strausfeld N. Mushroom bodies of the cockroach: their participation in place memory. J. Comp. Neurol. 1998b;402:520–537. doi:10.1002/(SICI)1096-9861(19981228)402:4<520::AID-CNE6>3.0.CO;2-K [PubMed] [Google Scholar]
- Morris R. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. doi:10.1016/0023-9690(81)90020-5 [Google Scholar]
- Rosano H, Webb B. Thoracic differentiation for the control of turning in the stick insect. Biol. Cybern. 2007;97:229–246. doi: 10.1007/s00422-007-0170-4. doi:10.1007/s00422-007-0170-4 [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Strambi A, Aouane A, Augier R, Rougon G, Cayre M. Suppression of adult neurogenesis impairs olfactory learning and memory in an adult insect. J. Neurosci. 2003;23:9289–9296. doi: 10.1523/JNEUROSCI.23-28-09289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szenher M. Visual homing in natural environments. In: Nehmzow U, Melhuish C, Witkowski M, editors. Proc. Towards Autonomous Robotic Systems. Department of Computing, Imperial College; London, UK: 2005. pp. 221–226. [Google Scholar]
- Tinbergen N, Kruyt W. Über die Orientierung des Bienenwolfes (Philanthus triangulum Fabr.) III. Die Bevorzugung bestimmter Wegmarken. Z. Vergl. Physiol. 1938;25:292–334. [Google Scholar]
- Weber T. Phonotaxis and visual orientation in Gryllus campestris: behavioural experiments. In: Lehrer M, editor. Sensory systems and communication in arthropods. Birkhäuser; Basel, Germany: 1990. pp. 377–386. [Google Scholar]
- Zampoglou M, Szenher M, Webb B. Adaptation of controllers for image-based homing. Adapt. Behav. 2006;14:381–399. doi:10.1177/1059712306072338 [Google Scholar]
- Zeil J, Hofmann M, Chahl J. Catchment areas of panoramic snapshots in outdoor scenes. J. Opt. Soc. Am. A. 2003;20:450–469. doi: 10.1364/josaa.20.000450. doi:10.1364/JOSAA.20.000450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional figures and explanations