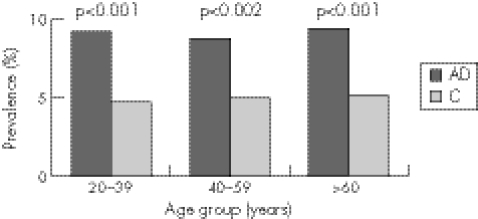Abstract
Background
Children with allergic diseases such as asthma and atopic dermatitis experience increased gastrointestinal symptoms. Further, physiological and histological abnormalities of the gastrointestinal tract in patients with allergic diseases have been reported. It is not certain whether adult patients experience increased gastrointestinal symptoms.
Methods
A retrospective, case–control study of 7235 adult (⩾20 years old) primary care patients was conducted. A general practitioner diagnosis of irritable bowel syndrome was used to serve as a marker of lower gastrointestinal symptoms. The prevalence of lower gastrointestinal symptoms was calculated in patients with asthma or allergic rhinitis and compared with that in patients with other chronic diseases (insulin‐dependent diabetes mellitus, osteoarthritis and rheumatoid arthritis) and with the remaining population.
Results
Gastrointestinal symptoms were significantly more common in patients with asthma (9.9%) as compared with patients with chronic diseases (4.9%; odds ratio (OR) 2.13, 95% confidence interval (CI) 1.39 to 2.56; p<0.002) or the remaining non‐asthmatic population (5.5%; OR 1.89, 95% CI 1.39 to 2.56; p<0.001). Gastrointestinal symptoms were also significantly more common in patients with allergic rhinitis (7.9%) as compared with patients with chronic diseases (4.9%; OR 1.66, 95% CI 1.02 to 2.7; p<0.05) and the remaining population (5.5%; OR 1.47, 95% CI 1.04 to 2.1; p<0.02). This phenomenon was independent of age, sex and inhaled asthma therapy in the case of patients with asthma.
Conclusions
Our findings support the hypothesis that lower gastrointestinal symptoms are more common in patients with allergic diseases such as asthma and allergic rhinitis.
Lower gastrointestinal symptoms such as diarrhoea and abdominal pain are common in children with allergic diseases such as asthma1 and atopic dermatitis.2 Although food allergies and rare organic gastrointestinal diseases such as eosinophilic gastroenteropathy are associated with atopic disease, it is unlikely that they alone would account for such symptoms. Histological abnormalities of the gastrointestinal tract in patients with allergic airway diseases have also been reported. Small bowel biopsy specimens from patients with asthma and allergic rhinitis show features in common with the inflammatory reaction observed in the airways, with accumulation of eosinophils, T cells, mast cells, macrophages and increased expression of proallergic cytokines, such as interleukin (IL)4 and IL5.3,4 Accumulation of eosinophils in the oesophageal mucosa has also been reported in patients with allergic rhinitis and asthma compared with controls.5 It is yet to be established whether these microscopic inflammatory changes influence gastrointestinal function. Interestingly, absorption studies with chromium 51‐labelled EDTA suggest a permeability defect of the gastrointestinal tract in patients with asthma.6
We hypothesised that lower gastrointestinal symptoms would be more prevalent in patients with asthma and allergic rhinitis. We conducted a retrospective, case–control study of community‐based patients by evaluating computerised records of patients from a large primary healthcare centre in the UK. This strategy allowed us to identify large numbers of patients with asthma and allergic rhinitis through specific diagnostic codes (James Read codes). We used a general practitioner diagnosis of irritable bowel syndrome (IBS) to serve as a marker for lower gastrointestinal symptoms. IBS has no specific features, and, typically, diagnosis depends on the presence of lower gastrointestinal tract symptoms in the absence of organic bowel disorders. Although criteria‐based definitions of IBS have been developed,7,8 it was not deemed necessary to use them strictly in this study, as we were simply using the diagnostic label to indicate the presence of lower gastrointestinal symptoms without an obvious organic cause.
Methods
We conducted a retrospective, case‐control study to determine the prevalence of gastrointestinal symptoms in patients with the allergic diseases asthma and allergic rhinitis in primary care. The study population comprised the entire adult population (aged ⩾20 years) of an urban primary healthcare centre in south Birmingham, UK, with 10 509 registered patients. Medical records for all patients in the practice were compiled on a computerised recording system. Diagnoses, including asthma, allergic rhinitis and IBS, were listed under specific James Read codes on the practice computer. Diagnoses were made by the general practitioners in the practice, or by doctors in the hospital following specialist referral based on the presence of typical symptom patterns or by specific tests (such as peak expiratory flow rate diary card monitoring in the case of asthma). Specific criteria regarding each individual diagnosis were not available for analysis. The Read code used to identify patients with asthma (H33..) included all subgroups of asthma (eg, atopic, non‐atopic, drug‐induced and exercised‐induced asthma). The Read code for rhinitis related only to patients with allergic rhinitis (H17..).
The computerised recording system allowed us to identify all patients in the practice diagnosed with asthma, allergic rhinitis and IBS. Two control groups were included in the study. The first control group comprised patients with chronic diseases, but not asthma or allergic rhinitis. These patients were diagnosed with insulin‐dependent diabetes mellitus (Read code C108.), osteoarthritis (N050.) and rheumatoid arthritis (N040.). The purpose of this group was to control for potential biases occurring owing to consulting behaviour among patients with chronic diseases (self‐referral bias). The second control group comprised the remaining population of patients from the same practice without asthma, allergic rhinitis or any of the other chronic disease groups from the “chronic disease” cohort. The four study groups (asthma, allergic rhinitis, chronic disease and control groups) were all from the same geographical area, and consequently were comparable with respect to socioeconomic, racial and environmental factors.
Patients with IBS were similarly defined (Read code J521) and subsequently the prevalence of IBS was calculated in patients from the asthma, allergic rhinitis, chronic disease and control groups. These diagnostic labels were lifetime diagnoses, and did not reflect current or active disease. It was not possible to identify particular symptom patterns (eg, diarrhoea predominance, constipation predominance) in patients with IBS.
We also conducted a subgroup analysis to determine the influence of age, sex and prescribed inhaled asthma therapy (in the case of patients with asthma) on the prevalence of gastrointestinal symptoms. To generate sufficient statistical power in subgroup analyses, we divided our study population into two groups: patients with allergic disease (comprising patients with asthma and allergic rhinitis) and a control group (comprising the chronic disease group and the remaining practice population). Specifically, we calculated the prevalence of gastrointestinal symptoms in patients with allergic disease as compared with the control group in 20‐year age bands (20–39, 40–59 and ⩾60 years) and in sex‐specific subgroups.
We also analysed the prevalence of IBS in patients with asthma according to the treatment for asthma prescribed within the preceding 6 months. Three treatment groups were defined. The first group comprised patients with asthma who had been prescribed short‐acting inhaled β2 adrenergic agonists (all preparations and inhalation devices containing salbutamol or terbutaline were included) in the preceding 6 months. Patients in this group had not been prescribed any inhaled corticosteroids during the same 6‐month period. The second group comprised patients with asthma who had been prescribed inhaled corticosteroids (beclomethasone diproprionate, fluticasone proprionate and budesonide of any inhaled preparation or dosage). Patients who had been prescribed both inhaled corticosteroids and β2 adrenergic agonists in the preceding 6 months were included in this group. The third group comprised patients with asthma who had not been prescribed either of these mainstay treatments. Less commonly prescribed drugs for asthma, including cromoglycates, anticholinergics and leucotriene antagonists, were not evaluated. Similarly, long‐acting β2 adrenergic agonists and oral corticosteroids were not evaluated. Patients with inflammatory bowel disease, coeliac disease, pancreatic insufficiency and colonic malignancy were excluded from the study. Data on the smoking status of patients in each study group were not available for analysis.
Statistical methods
Statistical differences in the prevalence of IBS in each of the study groups and in subgroup analyses were identified by calculating odds ratios (OR) with 95% confidence intervals (CI), and additionally by χ2 analysis. Demographic differences between the study groups were analysed by χ2 analysis for categorical data and by Student's t test for continuous data; significance was assumed when p<0.05.
Results
In all, 7235 adult (aged ⩾20 years) patients were identified, comprising 865 patients with asthma (prevalence 12%), 647 patients with allergic rhinitis (prevalence 8.9%) and 488 patients with other chronic diseases (insulin‐dependent diabetes mellitus, osteoarthritis and rheumatoid arthritis); the remaining 5235 patients served as an additional control group. Table 1 shows the characteristics of patients in the four groups. A number of relevant demographic differences were apparent between the four groups. The chronic disease cohort was significantly older in the asthmatic group, allergic rhinitis group and the remaining control group (p<0.001). There were equivalent proportions of female patients in the asthma, allergic rhinitis and chronic disease cohorts. However, there were significantly more women in each of these groups than in the control group (p<0.001). Overall, the prevalence of gastrointestinal symptoms in patients aged 20–39 years (5.6%), 40–59 years (5.7%) and ⩾60 years (6%) did not differ significantly.
Table 1 Patient demographics.
| Total patients | Male n (%) | Mean age (years) | Age range (years) | |
|---|---|---|---|---|
| Asthma | 865 | 361 (41.7)* | 48.2† | 20–104 |
| Allergic rhinitis | 647 | 267 (41.3)* | 42.2† | 20–91 |
| Chronic disease | 488 | 209 (42.8)* | 64.2† | 20–94 |
| Controls | 5235 | 2766 (52.4)* | 47.1† | 20–105 |
*There were significantly more women in the groups with asthma, allergic rhinitis and chronic disease than the control group (p<0.001).
†The chronic disease cohort was significantly older than the groups with asthma, allergic rhinitis and the remaining control group (p<0.001).
IBS, our marker of lower gastrointestinal symptoms, was significantly more common in adult patients with asthma (9.9%) as compared with adult patients with other chronic diseases (4.9%; OR 2.13, 95% CI 1.39 to 2.56; p<0.002) and also with the remaining non‐asthmatic population (5.5%; OR 1.89, 95% CI 1.39 to 2.56; p<0.001). Gastrointestinal symptoms were also significantly more common in patients with allergic rhinitis (7.9%) as compared with patients with other chronic diseases (4.9%; OR 1.66, 95% CI 1.02 to 2.7; p<0.05) and also with the remaining population (5.5%; OR 1.47, 95% CI 1.04 to 2.1; p<0.02).
We also analysed the prevalence of gastrointestinal symptoms in our study population in specified 20‐year age bands (20–39, 40–59 and >60 years). The overall prevalence of gastrointestinal symptoms in patients of all diagnoses in each of the 20‐year age bands did not differ significantly from one age band to the next (prevalence of IBS was 5.6% in the 20–39‐year age band, 5.7% in the 40–59‐yearage band and 6% in the ⩾60‐year age band). Similarly, in patients with allergic disease, the prevalence of gastrointestinal symptoms in each age band analysed was equivalent, suggesting that bowel symptoms are equally prevalent in all age groups and also in controls (fig 1). However, we showed an increased prevalence of gastrointestinal symptoms in patients with allergic disease in each 20‐year age band analysed when compared with controls. For this analysis, we pooled together patients with asthma and allergic rhinitis to generate sufficient statistical power. There was a significantly increased prevalence of gastrointestinal symptoms in patients with allergic disease in the 20–39‐year age group (OR 2.03, 95% CI 1.37 to 3.02; p<0.001), 40–59‐year age group (OR 1.83, 95% CI 1.17 to 2.86; p<0.002) and the ⩾60‐year age group (OR 1.93, 95% CI 1.22 to 3.05; p<0.001) as compared with the corresponding age group‐matched controls (fig 1). Separate analysis of patients with asthma and allergic rhinitis in the 20‐year age bands showed that gastrointestinal symptoms were significantly more common in patients with asthma in the 20–39‐year age group (10.2% v 4.7%, p<0.001), 40–59‐year age group (9.6% v 5%, p<0.003) and the ⩾60‐year age group (9.8% v 5.1%, p<0.003) as compared with controls. In patients with allergic rhinitis, the prevalence of gastrointestinal symptoms was significantly higher in the ⩾60‐year age group (8.7% v 5.1%, p<0.001) as compared with controls. Although the prevalence of gastrointestinal symptoms in patients with allergic rhinitis was increased in the age bands 20–39 years (7.4% v 4.7%, p<0.056) and 40–59 years (7.7% v 5%, p<0.09), this did not achieve statistical significance.
Figure 1 The prevalence of lower gastrointestinal symptoms in patients with allergic disease (AD) as compared with controls (C) in specific age bands.
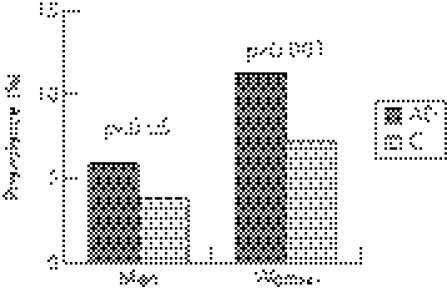
When assessed in sex‐specific groups, gastrointestinal symptoms were also more common in both female and male patients with allergic disease. There was a significantly increased prevalence of gastrointestinal symptoms in both female (OR 1.64, 95% CI 1.24 to 2.17; p<0.001) and male (OR 1.54, 95% CI 1 to 2.38; p<0.05) patients with allergic disease as compared with the controls of the same sex (fig 2). The overall prevalence of gastrointestinal symptoms was significantly increased among female patients of any diagnosis (8.2%) as compared with male patients of any diagnosis (4.2%; p<0.001).
Figure 2 The prevalence of lower gastrointestinal symptoms in male and female patients with allergic disease (AD) as compared with controls (C) of corresponding sex.
We also stratified patients with asthma according to the drugs prescribed within the preceding 6 months. We identified 499 patients who had been prescribed β2 adrenergic agonists, 268 patients who had been prescribed an inhaled corticosteroid preparation and 98 patients who had not been prescribed either of these treatments for asthma. There were no significant differences in the prevalence of gastrointestinal symptoms in patients with asthma who had been prescribed β2 agonists (10.4%), inhaled corticosteroids (9.3%) and no identified treatment for asthma (9.2%).
Discussion
Our study on a large primary care practice in the UK suggests that IBS, serving as a marker of lower gastrointestinal symptoms, is significantly more prevalent in adult patients with allergic diseases, such as asthma and allergic rhinitis, compared with controls. This increased prevalence of gastrointestinal symptoms in patients with allergic disease was consistently observed in patients of both sexes and in patients of all age groups studied, at least in the case of asthma. Our study is one of the biggest of its kind, dealing with a possible relationship between lower bowel symptoms and asthma, and is the only one in which the investigation has been extended to include allergic rhinitis.
Our data are congruent with other studies. In a study on 50 000 patients with asthma aged 10–79 years identified from the UK general practice research database,9 the cumulative prevalence of IBS was 2.5/1000 person‐years, compared with 2.0/1000 person‐years in a control population matched for age and sex, representing a significantly raised mean relative risk of 1.3 (95% CI 1.1 to 1.5). Interestingly, the risk of IBS was not significantly raised, compared with controls, in a subset of the patients with asthma taking systemic corticosteroids, an observation compatible with the hypothesis that systemic corticosteroid treatment might reduce IBS symptoms in some patient subgroups. In another study10 in which IBS was identified using a questionnaire based on the Rome II criteria, 41.3% of 150 patients with asthma, but only 22.3% of 130 patients who had other pulmonary diseases and 20.8% of 120 healthy controls, reported symptoms of IBS. As in our study, the prevalence of IBS was significantly higher in female patients, but did not significantly differ in patients with asthma stratified for treatment.
Conversely, in a study on 133 patients with IBS compared with 137 controls matched for age and sex, the prevalence of respiratory symptoms and diagnosed asthma was significantly higher in the patients with IBS as compared with controls.11 In this study, patients aged >50 years, smokers and patients with bowel disease of identifiable aetiology were excluded from analysis. In a small study comprising 11 patients with IBS, 11 with bowel disease of identifiable aetiology and 11 healthy controls, there was evidence of increased bronchial hyper‐responsiveness, a hallmark feature of asthma, following inhaled challenge with methacholine.12 However, much of this difference rested on enhanced bronchial responsiveness to inhaled methacholine at concentrations >16 mg/ml, which most investigators would regard as being outside the range compatible with a diagnosis of asthma. In a study of 3169 patients selected at random from a number of general practice registers responding to a validated postal questionnaire, the 1‐year prevalence of symptoms compatible with bronchial hyper‐responsiveness was 23% in patients with symptoms compatible with IBS but only 12% in patients not reporting symptoms of IBS.13 Similar significant differences in the prevalence of IBS were seen in a subset of the patients with asthma diagnosed by a doctor. These findings were not confounded by sex, and were also observed in a subset of the patients aged <40 years. These authors also noted an independent association of both IBS and gastro‐oesophageal reflux symptoms with asthma.
We consider that the consultation behaviour resulting in self‐referral bias was an unlikely confounding factor in our study, as we found that gastrointestinal symptoms were significantly more common in patients with asthma and allergic rhinitis as compared with our chronic disease control group that comprised patients with a range of chronic diseases. The consulting behaviour of the chronic disease cohort would probably be similar to that of patients with asthma and allergic rhinitis. In one of the studies referred to above,11 the association between patients reporting symptoms compatible with IBS and bronchial hyper‐responsiveness remained, irrespective of whether or not they had consulted their primary care physician in the preceding 2 years.
We considered the possibility that gastrointestinal symptoms might be a manifestation of adverse reactions to drugs prescribed to treat asthma and allergic rhinitis. We considered this unlikely, as the prevalence of IBS in patients with asthma treated with inhaled β2 agonists, inhaled corticosteroids or neither of these drugs did not significantly differ. We excluded patients taking systemic corticosteroids from our study; however, a previous study has suggested that such treatment is associated with fewer gastrointestinal symptoms. We also considered that diagnostic confusion of asthma with chronic obstructive pulmonary disease (COPD) was an unlikely confounding factor, as an increased prevalence of gastrointestinal symptoms was consistently observed in patients diagnosed with asthma across all age ranges analysed, and in particular the association remained even in the 20–39‐year age group, in whom COPD is uncommon. This possibility was also considered and ruled out in other studies,11,13 either by studying subsets of younger patients, unlikely to have symptoms such as breathlessness and wheeze resulting from COPD, or by excluding smokers from analysis.
In our study, 11% of patients with asthma not currently taking any drugs for asthma were identified. It is likely that these are patients whose symptoms are so mild that they no longer have a requirement for regular treatment, although they retain the lifelong diagnostic label of asthma. This reinforces the impression from our analysis of patients with asthma according to specific treatment, which suggests that bowel symptoms in patients with asthma are not directly related to the amount of anti‐asthma treatment (and by implication disease severity), but may reflect a lifelong propensity for abnormal function at both mucosal surfaces.
In our study, the four main study groups (patients with asthma, allergic rhinitis, chronic disease and controls) with important differences in demographic composition (table 1) require specific discussion. For example, the proportion of women in patients with asthma or allergic rhinitis was greater than in the control group. This is a potential source of bias, as IBS is well documented to occur more commonly in women (also shown in our study, fig 2). Reassuringly, IBS was significantly more common in patients with asthma and allergic rhinitis as compared with the chronic disease group which was composed of an equivalent proportion of female patients. Even more convincingly, we studied the prevalence of IBS in sex‐specific groups, in which IBS was observed to be more common in both men and women with allergic disease as compared with controls, suggesting that sex differences between the groups is not a likely source of significant bias. Another potentially relevant confounding issue is the increased mean age of patients in the chronic disease group as compared with patients with asthma or allergic rhinitis, especially as IBS is traditionally considered a disease of the young. These differences would probably not account for any significant confounding, because we specifically conducted an analysis of the prevalence of gastrointestinal symptoms in patients with allergic disease as compared with controls in predefined age‐specific categories. Our study suggested that gastrointestinal symptoms were significantly associated with allergic disease in young (20–39 years), middle‐aged (40–59 years) and elderly patients (⩾60 years) as compared with controls of equivalent age (fig 1). Further, there is increasing evidence that IBS is likely to be equally common in elderly patients.14,15 In our study, the total prevalence of gastrointestinal symptoms in patients of all diagnoses was statistically equivalent in patients aged 20–39, 40–59 and ⩾60 years (5.6%, 5.7% and 6.0%, respectively). These findings were similarly observed in patients subdivided into groups with allergic disease and controls (fig 1).
Another potential confounding factor in our study lies with the validity of non‐standardised definitions of asthma and IBS, which in this study relied on a general practitioner diagnosis. The reliability of the diagnostic criteria used may have varied between individual doctors in the participating practice. This is a valid criticism of the study, although we believe that it is unlikely to have invalidated the observed associations. The prevalence of asthma was within expected values. Further, it is arguable that asthma diagnosed by a doctor is at least as reliable as other methods of defining asthma, such as symptom scoring on questionnaire surveys, that have been used in large epidemiological studies, including some of those referred to earlier, which have all nevertheless produced broadly similar conclusions. Additionally, there is some evidence that a diagnosis of asthma can be reliably predicted from primary care prescription records alone,16 whereas in our study, patients with asthma were identified on the basis of a general practitioner diagnosis, which was corroborated by prescribing data.
Finally, it is also possible that potential biases may have been incurred by not accounting for smoking behaviour among our study population. Smoking may exacerbate symptoms of both IBS and asthma and might emphasise a potential association. We do not believe that this alone could account for the strength of the associations observed in this study. In particular, it is difficult to reconcile how this potential source of confounding could explain the association observed between allergic rhinitis and gastrointestinal symptoms.
In summary, we report an increased prevalence of lower bowel symptoms in patients with both asthma and allergic rhinitis. With the increasing interest in the concept of “united airways”,17 in which bronchial mucosal inflammation in asthma may influence nasal mucosal inflammation in rhinitis, and the increasing evidence of intestinal mucosal inflammation and altered permeability in patients with allergic disease,3,4,5,6 it is tempting to speculate that the term “united airways” may be extended to “united mucosa”, and that some of the gastrointestinal symptoms experienced by patients with allergic disease are a consequence of allergic inflammatory changes occurring in the gastrointestinal mucosa. This hypothesis is further reinforced by isolated suggestions that anti‐inflammatory treatment for asthma, such as systemic corticosteroids9 or leucotriene receptor antagonists18 may ameliorate symptoms in patients with IBS.
Further studies on the gastrointestinal tract of patients with allergic disease is merited to increase our understanding of how allergic immune reactions occurring in asthma and allergic rhinitis may involve other organ systems such as the gastrointestinal tract. In particular, evaluation of the factors that govern immune cell trafficking through mucosal tissue of both the respiratory and gastrointestinal tracts might provide insight into the mechanism of any potential association. The clinical effect and potential management of gastrointestinal tract symptoms in patients with allergic diseases such as asthma remains to be defined.
Acknowledgements
We thank Anne O'Brien for her help in performing computer searches and data collection.
Abbreviations
COPD - chronic obstructive pulmonary disease
IBS - irritable bowel syndrome
Footnotes
Competing interests: None declared.
References
- 1.Caffarelli C, Deriu F M, Terzi V.et al Gastrointestinal symptoms in patients with asthma. Arch Dis Child 200082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caffarelli C, Cavagni G, Deriu F M.et al Gastrointestinal symptoms in atopic eczema. Arch Dis Child 199878230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallaert B, Desreumaux P, Coplin M C.et al Immunoreactivity for interleukin 3 and 5 granulocyte/macrophage colony‐stimulating factor of intestinal mucosa in bronchial asthma. J Exp Med 19951821897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pires G V, Souza H S, Elia C C.et al Small bowel of patients with asthma and allergic rhinitis: absence of inflammation despite the presence of major cellular components of allergic inflammation. Allergy Asthma Proc 200425253–259. [PubMed] [Google Scholar]
- 5.Onbasi K, Sin A Z, Doganavsargil B.et al Eosinophil infiltration of the oesophageal mucosa in patients with pollen allergy during the season. Clin Exp Allergy 2005351423–1431. [DOI] [PubMed] [Google Scholar]
- 6.Benard A, Desreumaux P, Huglo D.et al Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol 1996971173–1178. [DOI] [PubMed] [Google Scholar]
- 7.Drossman D A, Thompson W G, Talley N H.et al Identification of subgroups of functional gastrointestinal disorders. Gastroenterol Int 19903159–172. [Google Scholar]
- 8.Manning A P, Thompson W G, Heaton K W.et al Towards positive diagnosis of the irritable bowel. BMJ 19782653–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huerta C, Garcia Rodriguez L A, Wallander M A.et al Risk of irritable bowel syndrome among asthma patients. Pharmacoepidemiol Drug Saf 20021131–35. [DOI] [PubMed] [Google Scholar]
- 10.Roussos A, Koursarakos P, Patsopoulos D.et al Increased prevalence of irritable bowel syndrome in patients with bronchial asthma. Respir Med 20039775–79. [DOI] [PubMed] [Google Scholar]
- 11.Yazar A, Atis S, Konca K.et al Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am J Gastroenterol 2001961511–1516. [DOI] [PubMed] [Google Scholar]
- 12.White A M, Stevens W H, Upton A R.et al Airway responsiveness to inhaled methacholine in patients with irritable bowel syndrome. Gastroenterology 199110068–74. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy T M, Jones R H, Hungin A P S.et al Irritable bowel syndrome, gastro‐oesophageal reflux, and bronchial hyper‐responsiveness in the general population. Gut 199843770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minocha A, Johnson W D, Abell T L.et al Prevalence, sociodemography, and quality of life of older versus younger patients with irritable bowel syndrome: a population‐based study. Dig Dis Sci 200651446–453. [DOI] [PubMed] [Google Scholar]
- 15. Ehrenpreis ED. Irritable bowel syndrome. 10% to 20% of older adults have symptoms consistent with diagnosis. Geriatrics 20056025–28. [PubMed] [Google Scholar]
- 16.Pont L G, van der Werf G T, Denig P.et al Identifying general practice patients diagnosed with asthma and their exacerbation episodes from prescribing data. Eur J Clin Pharmacol 200257819–825. [DOI] [PubMed] [Google Scholar]
- 17.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol 20031111171–1183. [DOI] [PubMed] [Google Scholar]
- 18.Fee W H. Irritable bowel syndrome helped by montelukast. Chest 20021221497. [DOI] [PubMed] [Google Scholar]