Abstract
Colon cancer is the second leading cause of cancer related death in American adults. The incidence and mortality are highest in African Americans (AAs) (incidence: 52 per 100 000) and lowest in American Hispanics (37 per 100 000). Comparative studies with Native Africans (<5 per 100 000) suggest that genetic susceptibility is an unlikely explanation and that environmental influences are to blame. Studies have suggested that risk is high because of excessive intakes of animal meat and fat products and differences in colonic bacterial metabolism, and that preventative and therapeutic management of colon cancer is compromised by the development of greater tumour virulence possibly resulting from disparities in educational and insurance status, screening behaviour, treatment patterns, social support, and access to and use of health care facilities. It should be possible to reduce the unacceptably higher rates of morbidity and mortality from colon cancer in AAs by dietary and lifestyle changes aimed at suppressing excessive intakes of animal meat and fat products, increasing the consumption of fresh fruit and vegetables, controlling energy balance, and by developing strategies to improve the availability, use and accessibility to health care resources.
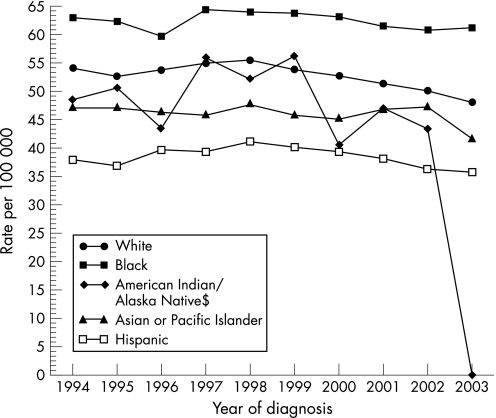
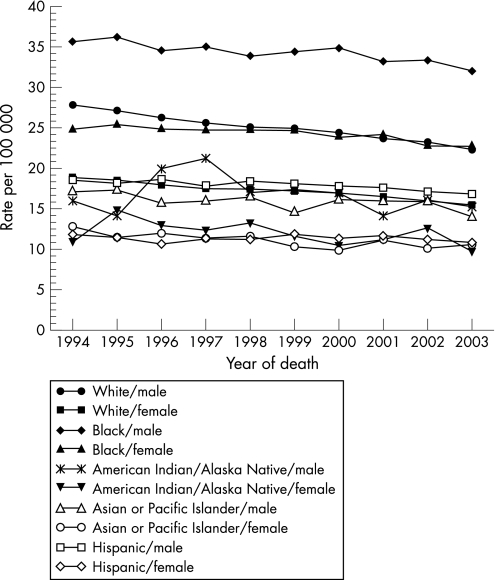
Colon cancer is one of the most common malignancies in developed countries. In the USA, it is the third most common cancer among men and women and, perhaps more importantly, is the second leading cause of cancer death for both sexes.1,2 The disease accounts for approximately 15% of all cancers diagnosed annually.3 There are important differences in its prevalence within racial and ethnic groups within the USA. African Americans (AAs) have the highest incidence and mortality: the age‐adjusted incidence rate for cancer of the colon and rectum (based on cases diagnosed in 2000–2003 from 17 Surveillance, Epidemiology and End Results (SEER) geographic areas) was 52.4 per 100 000 for men and women per year (fig 1) with 61.4 vs 72.9 per 100 000 men for white and black males and 44.7 vs 56.1 per 100 000 women for white and black females, respectively.2 The age‐adjusted death rate for colorectal cancer (based on patients who died in 2000–03 in the USA) was 19.8 per 100 000 men and women per year with 23.4 vs 33.4 per 100 000 men for white and black males and 16.2 vs 23.4 per 100 000 women for white and black females, respectively (fig 2).2
Figure 1 Surveillance, Epidemiology and End Results (SEER) age adjusted incidence rates by “expanded” race for colon and rectum cancer.2 All ages, both sexes. SEER 13 Registries for 1994–2003. Age‐adjusted to the 2000 US standard population.
Figure 2 Age‐adjusted total US mortality rates for colon and rectum cancer.2 For 1994–2003 by ‘expanded' race and sex. Age‐adjusted to the 2000 US standard population.
Based on rates from 2001–2003 both men and women face a lifetime risk of nearly 6% for the development of invasive colorectal cancer, with 50% of them being fatal.4 Colorectal cancer can be prevented and reduced by early detection and treatment. Its incidence and mortality rates have declined between 1985 and 2003 at an average annual rate of 1.6% and 1.8%, respectively, among US adults.1,2,5 When colorectal cancer is diagnosed at an early, localised stage (stage I and II), 5 year survival is 90%, but unfortunately only 37% of incident cases are diagnosed while still localised.5
Colorectal carcinoma usually arises from an adenomatous polyp and observational studies suggest that the adenoma‐to‐carcinoma sequence takes approximately 10 years.6 The hypothesis that invasive colorectal carcinoma develops from intermediate precancerous precursors is supported by pathologic, epidemiologic and observational clinical data, both in humans and in animal models. Although nearly 40% of Americans aged 50 years or older harbour adenomatous polyps, it is estimated that only 2% of adenomas will progress to cancer. Research suggests that the decline in rates may be due to an increased use of colonoscopic screening and polyp removal, which prevents the progression to cancer. However, these encouraging national trends are more evident in whites than in blacks. The only known race predilection is in AAs who bear a disproportionate burden of the morbidity and mortality.
Geographical variations
The incidence of colon cancer varies 20‐fold throughout the world's populations, with the highest rates seen in western countries and the lowest in developing countries.7 Migration studies have suggested that the variation can be accounted for by environmental rather than genetic differences. A study by Lichtenstein to estimate the magnitude of the genetic and environmental effects on susceptibility to sporadic colorectal cancer (comparisons of the concordance of cancer between monozygotic and dizygotic pairs of twins) pointed out that environment has a principal role in causing sporadic colorectal cancer as compared to hereditable causes (which accounted for approximately 35% of the risk).8 Although there are many differences in the environment that may influence colon cancer risk, the landmark epidemiological analyses performed by Doll and Peto lead them to suggest that 90% could be attributed to dietary factors.9
Evidence from animal and cross‐sectional studies has shown that several dietary factors are plausible explanations for population differences, and that rates of colon cancer increase for people migrating from low to high incidence areas. This point is well illustrated by a classic migrant study in which Hawaiian Japanese showed a progressive increase while, during the same time period, the incidence remained very low in Japan.10 Today, this change is occurring within Japan, where the incidence is increasing with progressive westernisation of the diet and lifestyle.11 Analysis of the dietary epidemiological data has led to the recognition that some dietary factors are associated with increased colon cancer risk, while others appear to be protective. Studies that have tried to explain the ethnic differences in AAs and Caucasians have found that, regardless of the ethnicity or energy consumption, high and frequent vegetable consumption was protective and consistent with a 20–50% reduction in risk. Further, in Caucasians, a high refined carbohydrate and red meat consumption (amount and frequency) was associated with a statistically significant twofold increased risk in non‐energy adjusted models.12
Perhaps the most consistent findings are that high levels of fat and meat intake promote risk, while the high consumption of cruciferous vegetables, fruits, fibre, folate and calcium suppress risk.13,14,15,16 The situation is, however, more complicated than this and the risk is determined by the balance between dietary intake, lifestyle and colonic bacterial metabolism, all of which modulate the internal environment of the colon and affect the regenerative epithelium.
African American versus Native African studies
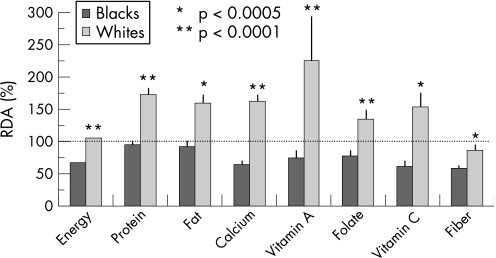
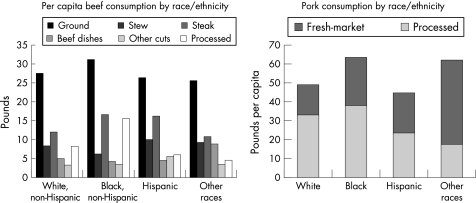
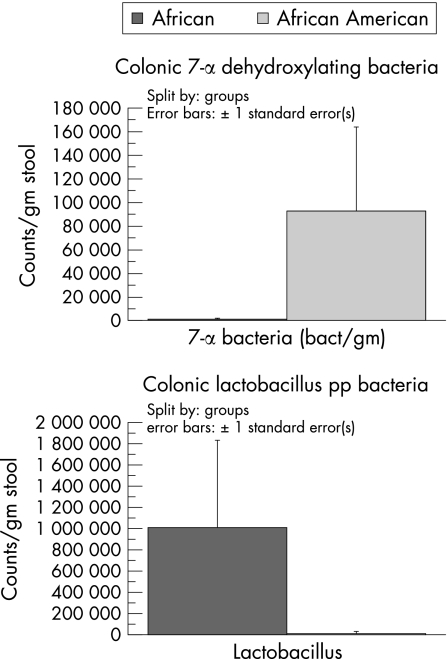
As an example of the complexity of the diet–colon cancer relationship, studies of ours have shown that the remarkably low risk of colon cancer in South African blacks (that is, <5/100 000) as opposed to African whites (>40/100 000) who consumed a typical “westernised” diet, was associated not with high consumption rates of fresh fruits and vegetables, antioxidant vitamins, folic acid, nor calcium, but with a low intake of animal proteins and fats (fig 3).17,18 This suggests that the aggravating effects of meat and saturated fat override the suppressive effects of vitamins and minerals, unlike the evidence that dietary folate may suppress the risk alcohol consumption has on colon cancer.19 More recent studies compared the diets of AAs to Caucasian Americans (CAs) and native Africans; they confirmed that cancer risk—as measured by epidemiological data and colonic epithelial proliferation rates—was higher in both American groups and associated with excessive intakes of animal proteins and fats (fig 4), as well as with differences in colonic bacterial flora (high clostridiae and low lactobacillus and methanogen species populations) (fig 5) and their rates of metabolism of undigested carbohydrate, as indicated by breath hydrogen or methane excretion after feeding.18 While the diet was more similar between AAs and CAs, with both groups consuming 2–4 times more animal proteins and fats, large scale national surveys have confirmed that the consumption of red meat and pork is higher in AAs than in other US racial subgroups (fig 4).
Figure 3 Comparison of dietary consumption patterns in black and white South Africans.17 RDA, recommended dietary allowance.
Figure 4 Per capita beef and pork consumption by race/ethnicity.
Figure 5 Results of faecal bacterial identification and quantisation from African Americans and native Africans.18
Our investigations also revealed potentially important differences in colonic bacterial flora, with a greater predominance of 7α dehydroxylating clostridiae species and a higher hydrogen generation from undigested carbohydrate. Clostridiae contain a 7α dehydroxylating enzyme, which enables the bacteria to convert primary bile salts to secondary salts, which have been shown to be carcinogenic in experimental models.20 Excessive hydrogen gas production is also toxic and may contribute to chronic inflammation, which is likely to promote neoplastic change after a lifetime of exposure.21 In contrast, we were unable to culture any lactobacillus organisms from AAs, which are anti‐inflammatory and thought to promote mucosal health.22
Although environmental factors play the major role in colorectal cancer risk, genetic susceptibility is clearly also important.23 Molecular epidemiological studies have shown that sporadic colorectal cancer only develops after an accumulation of several genetic changes, some of which are influenced by the diet. Genetic polymorphisms may interact with dietary components to modify cancer risk. A good example is dietary folic acid, which is essential for DNA synthesis and repair, and dietary deficiency may expose the mismatch repair polymorphisms that are associated with certain inherited forms of colon cancer, such as HNPCC.24 On the other hand, dietary factors may play a secondary role in the familial cancers associated with adenomatous polyposis, which result from germline mutations of the adenomatous polyposis coli gene.25
The observation that sections of a given population consuming a “westernised diet” have considerably higher colon cancer incidence rates than sections consuming a “traditional diet” has supported the view that the operative environmental factor is the diet. Given that both genetic and environmental factors play a role in colorectal cancer risk, failure to take into account both factors can lead to bias in the estimation of disease risk.
While the increased incidence of colon cancer in AAs is undoubtedly partly due to dietary factors, the increased mortality may also be attributed to disparities in access to health care and socioeconomic status. In the rest of the review, we will discuss these factors in more detail.
Reasons to explain racial differences in the morbidity and mortality of colon cancer
Cultural and socioeconomic
In 1973, Henschke et al published a landmark paper documenting the increasing disparities in cancer mortality between black and white Americans.26 The Civil Rights Movement and the paper by Henschke et al created a public interest in minority health and especially in minority cancer health. Twenty years after the publication of the paper by Henschke et al, an appreciation that there were continuing and widening disparities among black and white Americans in a number of diseases led to the enactment into law of the NIH (National Institutes of Health) Revitalization Act of 1993. This legislation required that all NIH‐sponsored phase III clinical trials include minorities and women in sufficient numbers such that a valid subset analysis could be done to ascertain differences in a treatment's effect among women and minorities and their subpopulations.27 The small number of black patients in the SEER–Medicare database poses a limitation in proper interpretation and examination of some of the potential regional variations in black–white disparity in chemotherapy receipt and overall colon cancer mortality. Screening behaviour has been cited as one of the reasons of higher incidence and mortality rate for blacks than for whites in the USA. These differences are seen among rural blacks and whites as compared to their urban counterparts regardless of stage of disease at diagnosis.28
An important factor in the increased morbidity in AAs with colon cancer is racial disparities in health care. A discussion of the myriad and complex causes and manifestations of racial and ethnic inequalities in the USA is beyond the scope of this review. The Institute of Medicine published an extensive report on the topic of disparities in health care in the USA and found that all racial and ethnic minorities receive a lower quality of health care, regardless of socioeconomic status, insurance coverage, age, or comorbid conditions. Some studies have found that there are residual confounding effects induced by comorbid conditions. For example, disparities in the rates of cardiovascular and other diseases may explain why the overall cancer survival remains disparate even when cancer specific survival is equal.29 European studies have linked these comorbid diseases to socioeconomic status and social deprivation within race.30,31 In the USA, race is a surrogate for socioeconomic status.29 A study that was done to address the socioeconomic gradients in cancer incidence among four mutually exclusive US racial/ethnic groups—Asian and Pacific Islander, black, Hispanic, and white—suggested that US cancer data should be stratified by socioeconomic position, along with race/ethnicity and gender, so as to improve cancer surveillance, research, and control.32
Some studies have sought to determine if colorectal cancer screening rates are different between blacks and whites (while controlling for potential confounders) and found that race was not significantly associated with current colorectal cancer screening status after adjusting for age, having a regular doctor, and participation in general medical exams. Race was also not a significant determinant of screening behaviour, and therefore did not explain the racial disparity in incidence or survival in these studies, while older age, having a regular doctor and participating in general medical exams were significant predictors of willingness to participate in colorectal cancer screening.33 Other studies have found that black patients did not see medical oncologists at different rates than whites, suggesting that physicians were referring black and white patients to medical oncologists in a comparable manner and that both black and white patients adhered to these recommendations and/or considered chemotherapy. However, black patients were less likely than white patients to initiate chemotherapy after this consultation.34
There is also evidence that treatment patterns vary throughout the USA and some studies have shown that there is differential cancer treatment by race. Black patients are less likely than white patients to receive screening tests, diagnostic tests, and a variety of treatments.35,36,37 The disparities have been demonstrated in the care of several cancer types. For example, Schrag et al found that after adjusting for sociodemographic, clinical, and environmental characteristics, black patients were statistically significantly less likely than white patients to receive recommended chemotherapy for stage III colon cancer.38 Black patients with colon cancer were less likely than white patients to undergo surgical resection (68% vs 78%), even after controlling for age, comorbidity, location of tumour, and extent of tumour.39 Studies that have examined the degree to which health systems factors explain black–white disparities in colon cancer care (for example, lower rate of receiving chemotherapy by black patients) have found varying results. Patient's age has been shown to be one of the factors associated with the black–white disparity in chemotherapy use. The youngest black Medicare beneficiaries experienced the greatest disparity in chemotherapy receipt. Older black patients had more similar care to older whites, in large part because of decreasing chemotherapy receipt with increasing age between both groups. Little disparity was explained by health systems; more was explained by illness severity, social support and environment.34 A study analysing the surgical resection of primary tumours in patients who present with stage IV colorectal cancer (surveillance, epidemiology, and end results data 1988–2000) found that the proportion of patients undergoing resection depended on the patient's age and race and the anatomical location of the primary tumour.40
The North Carolina Study Group examined the roles of religious involvement and social support in the risk of colon cancer, aetiology of colon cancer, and stage of disease at diagnosis.41 It reported that infrequent attendance at religious services (less than once per month) was associated with a regional/advanced stage of colon cancer at diagnosis in whites (odds ratio (OR) 1.67, 95% confidence interval (CI) 1.09 to 2.57; p for trend = 0.02), but not in blacks (OR 1.21, 95% CI 0.66 to 2.21; p for trend = 0.80). Among blacks, minimal emotional support was strongly associated with risk of colon cancer (OR 4.62, 95% CI 2.06 to 10.35; p for trend <0.001) and with both local (OR 3.69, 95% CI 1.08 to 12.69; p for trend <0.001) and advanced (OR 5.10, 95% CI 2.03 to 12.82; p for trend <0.01) disease. No associations between emotional support and risk of colon cancer or stage of disease were observed among whites.41 These results suggest that certain characteristics of social ties are associated with both risk of and prognostic indicators for colon cancer. Studies have also pointed to severity of illness, social support and environment as being more powerful factors than treating physicians or hospitals in explaining the disparities.
Surgical resection and length of stay in hospital also accounted for a substantial proportion of the black–white disparity. Black patients had longer lengths of stay than white patients. Length of stay could indicate underlying health status and/or the level of postoperative complication and thus provide a functional measure of health status at the time chemotherapy was being considered. Length of stay may also represent the level of home care support, because individuals with less support in their homes may require more care in the hospital before discharge. Poorer health status and less home care support could affect an oncologist's likelihood of recommending chemotherapy or a patient's perception of the ability to tolerate chemotherapy, although neither of these factors represents an absolute contraindication to receipt of chemotherapy. Educational status in a patient's residence explained a substantial proportion of the black–white disparity in chemotherapy use. A higher proportion of black patients lived in census tracts with lower high school graduate rates; these areas had lower chemotherapy rates.
Studies have also found an association between education level and use of recommended medical care, such as cancer screening and disease treatments.42,43,44 Lower educational attainment is associated with lower income. For persons under age 65 years, lower income is associated with less insurance coverage, which is highly correlated with receipt of less medical care, including cancer screening and treatment. Black patients have less supplemental coverage compared to whites that could affect co‐payment for outpatient chemotherapy, and this can affect acceptance rates among blacks without supplemental insurance.45 The literature suggests that black individuals are more likely than white individuals to have a fatalistic attitude toward medical illness; to experience stigma, fear, and denial related to a cancer diagnosis; to have an aversion to health care treatments such as surgery; to mistrust the health care system; and to have misperceptions about cancer that interfere with treatment.46,47 Black patients may place values on the projected benefits of chemotherapy that differ from those of white patients. It is also possible that black patients are more likely than white patients to misperceive chemotherapy for stage III colon cancer as palliative rather than adjunctive treatment. Oncologists may have difficulty communicating the benefits of chemotherapy alongside its risks in the context of these beliefs, perceptions, and experiences. Alternately, it is possible that medical oncologists caring for black patients may provide a lower quality of care than those caring for white patients. Oncologists may also view their black patients as less favourable chemotherapy candidates or may present chemotherapy less enthusiastically to black patients than to white patients.
Primary care physicians treating black patients have reported greater difficulty obtaining access for their patients to high quality subspecialists than physicians treating white patients. Several studies have shown that physicians are less likely to suggest or to provide recommended treatments to black patients than to white patients.48 Black patients perceive greater levels of racism or unfair treatment because of race in the health care system than white patients. They have also rated their medical visits as less participatory than have white patients. Colon cancer incidence also varies geographically and is related to aggregate socioeconomic factors, including physician density.49 Further studies with additional data are needed to examine potential regional variation in black–white disparity in chemotherapy receipt.
Tumour characteristics
For many years, health authorities have recognised the fact that black Americans with cancer experience higher mortality compared with white Americans at the same stage of disease. A number of researchers have postulated that biologically more aggressive tumours in blacks offer the most reasonable explanation for this disparity. Some studies that have looked at Kaplan–Meier survival probabilities according to race and tumour differentiation found no differences in the distribution of pathologic tumour stage between racial groups after stratifying by histologic tumour grade. They postulated that the increased mortality among AAs may not be attributable to an advanced pathologic stage of disease at diagnosis, but instead may be due to aggressive biologic features like high tumour grades.50 Among patients with high grade tumours, 54% of AAs and 21% of Caucasians died within the first year after surgery (p = 0.007). AAs with high grade tumours were three times (hazard ratio (HR) 3.05, 95% CI 1.32 to 7.05) more likely to die of colon carcinoma within 5 years post‐surgery, compared with caucasians with high grade tumours. There were no survival differences by race among patients with low‐grade tumours.51
However, data from other studies have shown that blacks were less likely to have poorly differentiated (grade 3) tumours and lymphoid reaction when compared with whites and that the stage at diagnosis accounted for more than half of the excess colon cancer mortality observed among blacks. Poverty and other socioeconomic conditions, general health status, tumour characteristics, and general patterns of treatment did not further explain the remaining survival disadvantage among blacks. These black/white differences remained statistically significant after adjusting for age, sex, metropolitan area, summary stage, socioeconomic status, body mass index, and health care access and utilisation. In addition, blacks were less likely to have high grade (grade 3) nuclear atypia, mitotic activity, and tubule formation, although these odds ratios did not reach a significance level of 0.05. Comparison by anatomical subsite showed that blacks had statistically significantly better differentiated tumours for cancers of the proximal and transverse colon but not for the distal. No racial differences were found for blood vessel and lymphatic invasion, necrosis, fibrosis, and mucinous type of histology. After adjusting for stage, more aggressive tumour characteristics do not explain the adverse survival differential in blacks. These variations in studies suggest that there may be racial differences in environmental exposure, and that the intensity and mode of delivery of carcinogen insult as well as host susceptibility may be influences by race and anatomical subsite.
Diet
Studies have reported that increasing age and tobacco use were linked independently to the presence of colonic aberrant crypt foci in predominantly AA populations. Folate, alcohol, and acetylsalicylic acid (aspirin) use did not influence the prevalence of these lesions in this population.52 Total energy intake is positively associated with colon cancer risk in both racial groups and, although there are some differences by race, high intakes of individual energy sources is also generally associated with a two‐ to threefold increase in risk in models not controlled for total energy. However, these associations largely disappeared when total energy is taken into account.53 Alcohol intake was not statistically significantly associated with colon cancer in either racial group. The North Carolina Colon Cancer Study that looked at the associations of micronutrients with colon cancer risk in AAs and whites reported lower mean micronutrient intakes in AAs, primarily due to larger contributions from dietary supplements in whites. In whites, high β‐carotene, vitamin C, and calcium intakes were associated with 40–60% reductions in colon cancer risk when contrasting highest to lowest quartiles in both energy adjusted and non‐energy adjusted models, while in AAs, vitamins C and E were strongly inversely associated with a reduced risk for colon cancer. Folate and lutein were not statistically significantly associated with colon cancer risk in either racial group.
These results suggested that at high intakes, micronutrients commonly found in plant and other foods (in particular, β‐carotene, vitamin C, and calcium in whites and vitamins C and E in AAs) exhibit independent associations consistent with 30–70% reductions in colon cancer risk.54 A positive association across all levels of exposure between red meat intake and colon cancer has been shown for 2‐amino‐3, 4, 8‐trimethylimidazo [4, 5‐f] quinoxaline (DiMeIQx), consistent with the hypothesis that heterocyclic amines (HCAs) may be among the aetiologically relevant compounds in red meat that are associated with colon cancer.55 HCAs, which are potent carcinogens in experimental animal models, are produced when red meat is burned (as when barbequed) and the high haem content of meat may also increase risk.56,57 In another study inverse associations between regular non‐steroidal anti‐inflammatory drug (NSAID) use and colon cancer were similar for AAs (OR 0.41, 95% CI 0.22 to 0.77) and whites (OR 0.48, 95% CI 0.28 to 0.83), but stronger for women than men. Inverse associations were slightly weaker for occasional versus regular NSAID use, but they were similar for aspirin and non‐aspirin NSAID use. These results add new knowledge, suggesting that the protective effect of NSAID use against colon cancer is similar among AAs and whites.58
Conclusions
The study of why AAs have higher incidence and mortality rates from colon cancer than other Americans helps us understand the complex interactions that exist between environmental factors such as diet, lifestyle and living conditions, and genetic susceptibility to cancer risk. Thus, given a relevant dietary exposure, the level of susceptibility to diet‐induced carcinogenesis may be the major determinant of variation in individual risk. This explains why not everybody who consumes a diet rich in experimentally proven carcinogens develops colon cancer, in the same way that not every smoker develops lung cancer. However, the association between the over‐consumption of specific dietary items and colon cancer within populations or ethnic groups indicates that dietary modification is likely to reduce risk, and that efforts should be made to educate the community to achieve this.
Despite the preoccupation with nutrition by the public and media in the USA, trends in food consumption have not been favourable, and the prevalence of obesity continues to rise. The average US diet is still too high in calories and fat and too low in fibre, cereals, fruits and vegetables. The fact that few short‐term dietary intervention studies have successfully decreased cancer risk and polyp formation should not be used to disregard the importance of a healthy diet and lifestyle, as cancer arises from a lifetime exposure to environmental carcinogens and the accumulation of a series of genetic aberrations. Rather, intensive effort needs to be given to educate parents on how best to feed their children.
With regard to the increased mortality from the disease, it is clear that tumour virulence can be promoted by environmental factors and ineffective or delayed treatment. State and community health care providers should be galvanised in their efforts to develop strategies aimed not only at prevention, but also early detection and comprehensive management of this acquired disease.
Abbreviations
AAs - African Americans
CAs - caucasian Americans
CI - confidence interval
HCA - heterocyclic amines
NIH - National Institutes of Health
NSAID - non‐steroidal anti‐inflammatory drug
OR - odds ratio
Footnotes
Competing interests: None declared.
References
- 1.Ries L A G, Eisner M P, Kosary C L.et al Hankey. SEER Cancer Statistics Reviews, 1973–1997. Bethesda, Maryland: National Cancer Institute, 2000
- 2.SEER http://seer.cancer.gov/csr/1975_2003/, based on November 2005 SEER data submission, posted to the SEER website 2006
- 3.Parkin D M, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 19994933–64. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Thomas A, Murray T.et al Cancer Statistics, 2002. CA Cancer J Clin 20025223–47. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention http://www.cdc.gov/cancer/colorctl/colorect.htm
- 6.O'Brien M J, Winawer S J, Waye J B. Colorectal polyps. In: Winawer SJ, ed. Management of gastrointestinal diseases. New York: Gower Medical, 1992
- 7.World Cancer Research Fund and American Institute for Cancer Research Colon, rectum. In: Food, nutrition and prevention of cancer: a global perspective Washington, DC: World Cancer Research Fund and American Institute for Cancer Prevention, 1997216–251. [DOI] [PubMed]
- 8.Lichtenstein P, Holm N V, Verkasalo P K.et al Environmental and heritable factors in the causation of cancer‐‐analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 200034378–85. [DOI] [PubMed] [Google Scholar]
- 9.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981661191–1308. [PubMed] [Google Scholar]
- 10.Haenszel W, Kurihara M. Studies of Japanese migrants: mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst 1968404. [PubMed] [Google Scholar]
- 11.Lee J A. Recent trends of large bowl cancer in Japan compared to United States and England and Wales. Int J Epidemiology 19765187. [DOI] [PubMed] [Google Scholar]
- 12.Satia‐Abouta J, Galanko J A, Martin C F.et al Food groups and colon cancer risk in African‐Americans and Caucasians. Int J Cancer 2004109728–736. [DOI] [PubMed] [Google Scholar]
- 13.Willet W C, Stampfer M J, Colditz G A.et al Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 19903231664. [DOI] [PubMed] [Google Scholar]
- 14.Sellers T A, Bazyk A E, Bostick R M.et al Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States). Cancer Causes Control 19989357–366. [DOI] [PubMed] [Google Scholar]
- 15.Pietinen P, Malila N, Virtanen M.et al Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 199910387–396. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Stampfer M J, Colditz G A.et al Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst 19928491–98. [DOI] [PubMed] [Google Scholar]
- 17.O'Keefe S J D, Kidd M, Noel G.et al The rarity of colon cancer in Africans is associated with low animal product consumption, not fiber. Am J Gastroenterology 1999941373–1380. [DOI] [PubMed] [Google Scholar]
- 18.O'Keefe S J, Chung D, Mahmoud N.et al Why do African Americans get more colon cancer than native Africans? J Nutr 2007137175S–82S. [DOI] [PubMed] [Google Scholar]
- 19.Giovannuci E. Modifiable risk factors of colon cancer. Gastroenterol Clin North Am 200231925–943. [DOI] [PubMed] [Google Scholar]
- 20.Watabe J, Bernstein H. The mutagenicity of bile acids using a fluctuation test. Mutat Res 198515845–51. [DOI] [PubMed] [Google Scholar]
- 21.Gibson G R, MacFarlane G T, Cummings J H. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut 199334437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pochapin M. The effect of probiotics on C difficile diarrhea. Am J Gastroenterol 200095(Suppl)S11–S13. [DOI] [PubMed] [Google Scholar]
- 23.Mac Lennan R. Diet and colorectal cancer. Int J Cancer 1997(Suppl 10)10–12. [DOI] [PubMed]
- 24.Novakovic P, Stempak J M, Sohn K J.et al Effects of folate deficiency on gene expression in the apoptosis and cancer pathways in colon cancer cells. Carcinogenesis 200627916–924. [DOI] [PubMed] [Google Scholar]
- 25.Burt R W. Familial risk and colorectal cancer. Gastroenterol Clin North Am 199625793–803. [DOI] [PubMed] [Google Scholar]
- 26.Henschke U K, Leffall L D, Jr, Mason C H.et al Reinhold AW, Schneider RL, White JE. Alarming increase of the cancer mortality in the U.S. black population (1950–1967). Cancer 197331763–768. [DOI] [PubMed] [Google Scholar]
- 27.Freedman L S, Simon R, Foulkes M A.et al Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993—the perspective of NIH clinical trialists. Control Clin Trials 199516277–85 discussion 2869, 293309. [DOI] [PubMed] [Google Scholar]
- 28.Kinney A Y, Harrell J, Slattery M.et al Rural‐urban differences in colon cancer risk in blacks and whites: the North Carolina Colon Cancer Study. J Rural Health 200622124–130. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman J S, Cooper R S, McGee D L. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology 19978621–628. [PubMed] [Google Scholar]
- 30.Faggiano F, Partanen T, Kogevinas M.et al Socioeconomic differences in cancer incidence and mortality. IARC Sci Publ 199713865–176. [PubMed] [Google Scholar]
- 31.Marmot M, Feeney A. General explanations for social inequalities in health. IARC Sci Publ 1997138207–228. [PubMed] [Google Scholar]
- 32.Krieger N, Quesenberry C, Jr, Peng T.et al Social class, race/ethnicity, and incidence of breast, cervix, colon, lung, and prostate cancer among Asian, Black, Hispanic, and White residents of the San Francisco Bay Area, 1988–92 (United States). Cancer Causes Control 199910525–537. [DOI] [PubMed] [Google Scholar]
- 33.Fisher D A, Dougherty K, Martin C.et al Race and colorectal cancer screening: a population‐based study in North Carolina. North Carolina Med J 200465(1) [PubMed] [Google Scholar]
- 34.Baldwin L M, Dobie S A, Billingsley K.et al Explaining black‐white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst 2005971211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon L F, Jr, Wolfe R A, Huang S.et al Racial and gender variation in use of diagnostic colonic procedures in the Michigan Medicare population. Med Care 199937712–717. [DOI] [PubMed] [Google Scholar]
- 36.Gornick M E, Eggers P W, Reilly T W.et al Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med 1996335791–799. [DOI] [PubMed] [Google Scholar]
- 37.McBean A M, Gornick M. Differences by race in the rates of procedures performed in hospitals for Medicare beneficiaries. Health Care Finance Rev 19941577–90. [PMC free article] [PubMed] [Google Scholar]
- 38.Schrag D, Cramer L D, Bach P B.et al Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 200193850–857. [DOI] [PubMed] [Google Scholar]
- 39.Cooper G S, Yuan Z, Stange K C.et al Use of Medicare claims data to measure county‐level variations in the incidence of colorectal carcinoma. Cancer 199883673–678. [DOI] [PubMed] [Google Scholar]
- 40.Cook A D, Single R, McCahill L E. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 200512637–645. [DOI] [PubMed] [Google Scholar]
- 41.Kinney A Y, Bloor L E, Dudley W N.et al Roles of religious involvement and social support in the risk of colon cancer among Blacks and Whites. Am J Epidemiology 20031581097–1107. [DOI] [PubMed] [Google Scholar]
- 42.Nadel M R, Blackman D K, Shapiro J A.et al Are people being screened for colorectal cancer as recommended? Results from the National Health Interview Survey. Prev Med 200235199–206. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman‐Goetz L, Breen N L.et al The impact of social class on the use of cancer screening within three racial/ethnic groups in the United States. Ethn Dis 1998843–51. [PubMed] [Google Scholar]
- 44.Jepson C, Kessler L G, Portnoy B.et al Black‐white differences in cancer prevention knowledge and behavior. Am J Public Health 199181501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Super N. Medigap: prevalence, premiums, and opportunities for reform. NHPF Issue Brief 20021–23. [PubMed]
- 46.Advani A S, Atkeson B, Brown C L.et al Barriers to the participation of African‐American patients with cancer in clinical trials: a pilot study. Cancer 2003971499–1506. [DOI] [PubMed] [Google Scholar]
- 47.Gregg J, Curry R H. Explanatory models for cancer among African‐American women at two Atlanta neighborhood health centers: the implications for a cancer‐screening program. Soc Sci Med 199439519–526. [DOI] [PubMed] [Google Scholar]
- 48.Sedlis S P, Fisher V J, Tice D.et al Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs Medical Center. J Clin Epidemiol 199750899–901. [DOI] [PubMed] [Google Scholar]
- 49.Shipp M P, Desmond R, Accortt N.et al Population‐based study of the geographic variation in colon cancer incidence in Alabama: relationship to socioeconomic status indicators and physician density. South Med J 2005981076–1082. [DOI] [PubMed] [Google Scholar]
- 50.Alexander D, Jhala N, Chatla C.et al High‐grade tumor differentiation is an indicator of poor prognosis in African Americans with colonic adenocarcinomas. Cancer 20051032163–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satia J A, Campbell M K, Galanko J A.et al Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev 2004131022–1031. [PubMed] [Google Scholar]
- 52.Moxon D, Raza M, Kenney R.et al Relationship of aging and tobacco use with the development of aberrant crypt foci in a predominantly African‐American population. Clin Gastroenterol Hepatol 20053271–278. [DOI] [PubMed] [Google Scholar]
- 53.Satia‐Abouta J, Galanko J A, Potter J D, for the North Carolina Colon Cancer Study et al Associations of total energy and macronutrients with colon cancer risk in African Americans and whites: results from the North Carolina Colon Cancer Study. Am J Epidemiology 2003158951–962. [DOI] [PubMed] [Google Scholar]
- 54.Satia‐Abouta J, Galanko J A, Martin C F.et al Associations of micronutrients with colon cancer risk in African Americans and whites: results from the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev 200312747–754. [PubMed] [Google Scholar]
- 55.Butler L M, Sinha R, Millikan R C.et al Martin. Heterocyclic amines, meat intake, and association with colon cancer in a population‐based study. Am J Epidemiol 2003157434–445. [DOI] [PubMed] [Google Scholar]
- 56.Norat T, Lukanova A, Ferrari P.et al Meat consumption and colorectal cancer risk: dose response meta‐analysis of epidemiological studies. Intl J Cancer 200298241–256. [DOI] [PubMed] [Google Scholar]
- 57.Willett W C, Stampfer M J, Colditz G A.et al Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 19903231664–1672. [DOI] [PubMed] [Google Scholar]
- 58.Sansbury L B, Millikan R C, Schroeder J C.et al Use of nonsteroidal anti‐inflammatory drugs and risk of colon cancer in a population‐based, case‐control study of African Americans and Whites. Am J Epidemiol 2005162548–558. [DOI] [PubMed] [Google Scholar]