Abstract
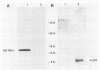
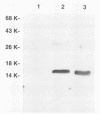
A peptidoglycan-associated lipoprotein of about 15 kilodaltons was purified from the outer membranes of Haemophilus influenzae by using nondenaturing detergents. To assess its vaccine potential, rabbit antiserum to the purified protein was obtained. The antiserum was specific for the peptidoglycan-associated lipoprotein in whole cell lysates of H. influenzae and was bactericidal for H. influenzae types a, b, d, e, and f and for 181 of 182 H. influenzae type b clinical strains isolated in widely dispersed geographic areas. The antibody protected infant rats from challenge with each of five clinical H. influenzae type b isolates and was additive to and did not interfere with bactericidal and protective activities of antibody against the type b capsule. These data indicate that the purified peptidoglycan-associated lipoprotein is a potentially valuable vaccine candidate for H. influenzae type b disease and may enhance the effectiveness of preexisting anticapsular antibody.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Pichichero M. E., Insel R. A. Immunization of 2-month-old infants with protein-coupled oligosaccharides derived from the capsule of Haemophilus influenzae type b. J Pediatr. 1985 Sep;107(3):346–351. doi: 10.1016/s0022-3476(85)80504-7. [DOI] [PubMed] [Google Scholar]
- Branefors P., Dahlberg T. Serum bactericidal effect on capsulated and non-capsulated Haemophilus influenzae. Acta Pathol Microbiol Scand C. 1980 Feb;88(1):47–56. doi: 10.1111/j.1699-0463.1980.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Tsang P., Larose Y. Parameters affecting ascites tumour formation in mice and monoclonal antibody production. J Immunol Methods. 1984 Jul 6;71(2):265–272. doi: 10.1016/0022-1759(84)90073-5. [DOI] [PubMed] [Google Scholar]
- Evans F. O., Jr, Sydnor J. B., Moore W. E., Moore G. R., Manwaring J. L., Brill A. H., Jackson R. T., Hanna S., Skaar J. S., Holdeman L. V. Sinusitis of the maxillary antrum. N Engl J Med. 1975 Oct 9;293(15):735–739. doi: 10.1056/NEJM197510092931502. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Anderson P. Serum factor-dependent resistance of Haemophilus influenzae type b to antibody to lipopolysaccharide. J Infect Dis. 1985 May;151(5):869–877. doi: 10.1093/infdis/151.5.869. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Scales R., Warren K. A., Frank M. M., Rice P. A. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. 1985 Nov;76(5):1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Campagnari A. A., Nelson M. B., Apicella M. A. Antigenic characterization of the P6 protein of nontypable Haemophilus influenzae. Infect Immun. 1986 Dec;54(3):774–779. doi: 10.1128/iai.54.3.774-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Rice P. A., Nelson M. B., Dudas K. C., Apicella M. A. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Invest. 1986 Oct;78(4):1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Nelson M. B., Dudas K. C., Mylotte J. M., Apicella M. A. Identification of a specific epitope of Haemophilus influenzae on a 16,600-dalton outer membrane protein. J Infect Dis. 1985 Dec;152(6):1300–1307. doi: 10.1093/infdis/152.6.1300. [DOI] [PubMed] [Google Scholar]
- Norden C. W. Variable susceptibility of Hemophilus influenzae, type B strains to serum bactericidal activity. Proc Soc Exp Biol Med. 1972 Jan;139(1):59–61. doi: 10.3181/00379727-139-36076. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Smail E. H., Jones J. M. Demonstration and solubilization of antigens expressed primarily on the surfaces of Candida albicans germ tubes. Infect Immun. 1984 Jul;45(1):74–81. doi: 10.1128/iai.45.1.74-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Baker C. J., Quinones F. J., Hollis D. G., Weaver R. E., Wiss K. Nontypable Haemophilus influenzae (biotype 4) as a neonatal, maternal, and genital pathogen. Rev Infect Dis. 1983 Jan-Feb;5(1):123–136. doi: 10.1093/clinids/5.1.123. [DOI] [PubMed] [Google Scholar]