Abstract
Heparin‐induced thrombocytopenia (HIT) is a potentially devastating immune mediated adverse drug reaction caused by the emergence of antibodies that activate platelets in the presence of heparin. Despite thrombocytopenia, bleeding is rare; rather, HIT is strongly associated with thromboembolic complications involving both the arterial and venous systems. A number of laboratory tests are available to confirm the diagnosis; however, when HIT is clinically suspected, treatment should not be withheld pending the result. Fortunately, therapeutic strategies have been refined, and new and effective therapeutic agents are available. Treatment options are focused on inhibiting thrombin formation or direct thrombin inhibition. Warfarin should not be used until the platelet count has recovered.
Heparin is widely used for thromboprophylaxis or treatment in many clinical situations, including cardiovascular and orthopaedic surgery and invasive procedures, acute coronary syndromes, venous thromboembolism, atrial fibrillation, peripheral occlusive disease, dialysis, and during extracorporeal circulation.1 One third of hospitalised patients in the USA, or about 12 million a year, receive heparin.2 Heparin‐induced thrombocytopenia (HIT) is the most important and most frequent drug‐induced type of thrombocytopenia. It is associated with significant morbidity and mortality if unrecognised. Unfortunately, because thrombocytopenia is common in hospitalised patients and can be caused by a variety of factors,3 HIT often remains unrecognised and undiagnosed.
HIT may develop in two distinct forms: type I and type II. HIT type I (also known as heparin‐associated thrombocytopenia) is a non‐immunologic response to heparin treatment, mediated by a direct interaction between heparin and circulating platelets causing platelet clumping or sequestration. HIT type I affects up to 10% of patients, usually occurs within the first 48–72 h after initiation of heparin treatment, and is characterised by a mild and transient thrombocytopenia (rarely <100 000/mm3), often returning to normal within 4 days once the heparin is withdrawn.4 No laboratory tests are required to diagnose HIT type I, and it is not associated with an increased risk of thrombosis, whereas HIT type II is immune‐mediated and associated with a risk of thrombosis. It has recently been proposed that the term “HIT type I” be changed to “non‐immune heparin associated thrombocytopenia” and that the term “HIT type II” be changed to “HIT” to avoid confusion between the two syndromes.5
In this review, we briefly analyse the main characteristics of the clinically relevant, immune‐mediated, HIT type II, focusing particularly on the epidemiology, pathophysiology, clinical manifestations and treatment of this syndrome. For simplicity, in this review the term HIT refers only to HIT type II.
Pathophysiology
Low molecular weight heparins (LMWH), molecular weight 2000–10 000 Daltons (Da), are produced by chemical or enzymatic processes from unfractionated heparins (UFH).6 UFH are heterogenous mixture of negatively charged, sulfated glycosaminoglycan (3000–30 000 Da) derived from animal sources.6 HIT is caused by the formation of antibodies that activate platelets following heparin administration.7 The principal antigen is a complex of heparin and platelet factor 4 (PF4), a small positively charged molecule of uncertain biological function, normally found in α‐granules of platelets.8 Heparin's high affinity for PF4 depends upon molecular weight, chain length and its degree of sulfation, which explains the differences in incidence of HIT observed with different heparins.8
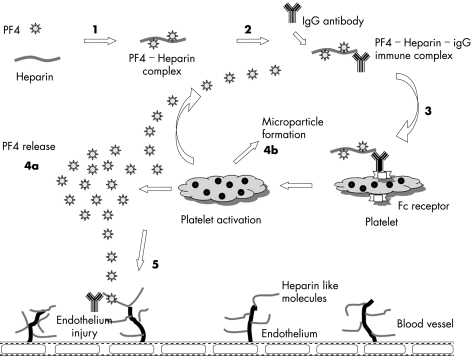
When heparin binds with PF4, it undergoes a conformational change and becomes immunogenic (fig 1), leading to the generation of heparin–PF4 antibodies (HIT antibodies), most frequently IgG.9 The heparin–PF4–IgG multimolecular immune complex then activates platelets via their FcγIIa receptors, causing the release of prothrombotic platelet‐derived microparticles, platelet consumption, and thrombocytopenia.9 These microparticles in turn promote excessive thrombin generation, frequently resulting in thrombosis. The antigen–antibody complexes also interact with monocytes, leading to tissue factor production, and antibody‐mediated endothelial injury may occur. Both of these latter processes may contribute further to the activation of the coagulation cascade and thrombin generation.
Figure 1 Pathophysiology of HIT. (1) Heparin binds with PF4 and act as immunogens. (2) IgG antibody thus produced forms PF4–heparin–IgG multimolecular complex. (3) The complex then binds via Fc receptor to platelets and activates them (4a) activated platelet releases additional PF4 and (4b) prothrombotic microparticles. (5) Immune complex interacts with endothelial cells and promotes immune mediated endothelial damage.
Polymorphism in the platelet FcγIIa receptors plays an important role in determining platelet reactivity and different FcγIIa phenotypes may be responsible for the risk variability to develop HIT10; however, no correlation between platelet glycoprotein and clotting factor polymorphisms and the risk of developing HIT has been identified.11
Thrombocytopenia in HIT is largely due to the clearance of activated platelets and antibody‐coated platelets by the reticulo‐endothelial system.1
Incidence
Patients of any age, receiving any type of heparin at any dose by any route of administration, are at risk of developing HIT antibodies, but they will all not necessarily develop the clinical syndrome of HIT. It is important to distinguish the frequency of antibody detection, antibody formation with thrombocytopenia (HIT), and antibody formation with thrombosis.
Up to 8% of patients receiving heparin are at risk to develop HIT antibodies,12 but only 1–5% on heparin will progress to develop HIT with thrombocytopenia13 and subsequently one third of them may suffer from arterial and/or venous thrombosis.14 In general, incidence of HIT is greater with bovine versus porcine heparin (all heparins used in the UK are of porcine origin), with unfractionated heparin versus LMWH (although it must be highlighted that antibodies developing in patients receiving UFH frequently crossreact with LMWH), and in post‐surgical (cardiac > orthopaedic > vascular > general) versus medical (0.8%) or obstetric patients.15 In orthopaedic patients given subcutaneous prophylactic heparin, the incidence is approximately 5% with UFH and 0.5% with LMWH.16 Hospital‐wide surveillance studies of 32–36 months suggest that HIT occurred in 1.2% of all patients who received heparin for >4 days.17
The risk to develop HIT depends upon various factors (as discussed above) and absence of thrombocytopenia after repeated and prolonged exposure to heparin does not necessarily eliminate the risk but indicates that such patients are at low risk of developing HIT, although there are limited data to support the argument.
Clinical features
The thrombocytopenia of HIT is typically of moderate severity, with median platelet counts ranging between 50–80×109/l.18 It may be absolute (that is, <150×109/) or relative (that is, a drop of 50% or more) compared with the pre‐heparin value, although the nadir may remain >150×109/l. Severe thrombocytopenia (platelets <15×109/l) is unusual. This fall in platelet count typically starts 5–14 days after initiation of heparin,15 but onset may be rapid or delayed. If a patient has circulating heparin–PF4 antibodies from a recent heparin exposure, the platelet count may drop within minutes or hours, resulting in rapid‐onset HIT.19 Conversely, in delayed‐onset HIT,20 the thrombocytopenia is delayed for several days, possibly up to 3 weeks, and only becomes evident after heparin treatment has already been stopped.
The platelet count starts to rise within 2–3 days and usually returns to normal within 4–10 days after cessation of heparin treatment, and it takes another 2–3 months for antibodies to disappear.13 In patients with persistent or worsening thrombocytopenia despite absolute discontinuation of heparin, other possible causes of thrombocytopenia must be considered and investigated; a decision to recommence heparin should be made after careful risk–benefit analysis in individual patients.
Complications
Despite thrombocytopenia, bleeding is rather rare.21 HIT is strongly associated with thromboembolic complications which can be venous, arterial, or both, and include deep venous thrombosis, pulmonary embolism, myocardial infarction, thrombotic stroke, and occlusion of limb artery requiring amputation.18 However, the type and site of thrombosis depends on the patient's clinical profile. Deep vein thrombosis and pulmonary embolism occur more frequently in postoperative patients.12 Similarly, the presence of a central venous catheter is associated with increased upper‐extremity venous thrombosis.1 Thrombosis of the cerebral venous sinuses and disseminated intravascular coagulation has also been reported.22 In contrast, arterial thrombosis occurs more frequently than venous thrombosis in HIT patients receiving heparin for cardiovascular diseases.23
Other complications of HIT include necrotising skin lesions at the injection site in 10–20% of patients, and acute systemic reactions, characterised by fever, chills, hypertension, tachycardia, chest pain and dyspnoea, in up to 25% of patients with circulating HIT antibodies.21 Thrombosis in HIT is associated with a mortality of approximately 20–30%, with an equal percentage of patients becoming permanently disabled by amputation, stroke or other causes.4
Risk factors
Risk factors suggestive of adverse outcomes in HIT include severity of the thrombocytopenia,24 and lower platelet counts which are associated with poor outcome, malignancy,25 and gender. Females are more likely to suffer thrombotic stroke as an outcome of HIT.26
Diagnosis
The diagnosis of HIT remains a clinical one, supported by confirmatory laboratory testing. The criteria for diagnosis of HIT include:
normal platelet count before the commencement of heparin
thrombocytopenia defined as a drop in platelet count by 30% to <100×109/l or a drop of >50% from the patient's baseline platelet count
onset of thrombocytopenia typically 5–10 days after initiation of heparin treatment, which can occur earlier with previous heparin exposure (within 100 days)
acute thrombotic event
the exclusion of other causes of thrombocytopenia
the resolution of thrombocytopenia after cessation of heparin
HIT antibody seroconversion
The “4 T's” of HIT (table 1), as suggested by Warkentin and et al,27 has been recommended by British haemostasis and thrombosis task force for standards in haematology to be used to assess patients with clinical suspicion of HIT.
Table 1 Estimating the pre‐test probability of heparin induced thrombocytopenia: the “4 T's”.
| Category | 2 points | 1 point | 0 point |
|---|---|---|---|
| Thrombocytopenia | >50% fall, or nadir of 20–100×109/l | 30–50% fall, or nadir of 10–19×109/l | 30% fall or nadir <10×109/l |
| Timing of platelet count fall | Days 5 to 10, or ⩽1 day if heparin exposure within past 30 days | >Day 10 or unclear (but fits with HIT), or ⩽1 day if heparin exposure within past 30–100 days | ⩽1 day (no recent heparin) |
| Thrombosis or other sequelae | Proven thrombosis, skin necrosis, or, after heparin bolus, acute systemic reaction | Progressive, recurrent, or silent thrombosis; erythematous skin lesions | None |
| Other cause for thrombocytopenia | None evident | Possible | Definite |
*Points assigned in each of four categories are totalled, and the pre‐test probability of HIT by total points is as follows: 6 to 8 = high, 4 to 5 = intermediate; 0 to 3 = low. Adapted with permission from Warkentin et al. Hematology/the education program of the American Society of Hematology. Copyright 2003, American Society of Hematology.
HIT is a potentially life‐threatening condition; it should be considered with priority among the possible causes of thrombocytopenia with or without thrombosis in a patient receiving heparin, particularly in a patient recently discharged from hospital who presents with thromboembolism.28
Laboratory testing
It is recommended that heparin–PF4 antibody testing, in patients with clinical suspicion of HIT, should be considered. However, results of laboratory tests may not be available for hours to days after being requested. Therefore, it is very important that initiation of proper treatment must never be delayed pending laboratory confirmation of HIT.
HIT antibodies can be demonstrated in vitro by functional tests and immunoassays.21 Functional tests measure platelet activity in the presence of the patient's serum and heparin. These include heparin‐induced platelet aggregation (HIPA), the serotonin release assay (SRA) and flow cytometric assays that detect platelet microparticle release.
Although the HIPA test (sensitivity 35–85%) is easier to perform and thus more commonly used, the SRA is 95% sensitive and specific, particularly when washed platelets are used, but is more complex, technically demanding and might not readily be available in most laboratories.21 The immunoassays utilise immuno‐enzymatic tests (ELISA) to detect the HIT antibody that binds to the PF4–heparin complex. Immunoassays have high sensitivity of 80–100% but low specificity.21 They are technically easier to perform than the functional assays but detect antibodies that do not elicit HIT (false positives) and has decreased specificity in certain populations such as cardiac surgery patients.
No single assay has 100% sensitivity and specificity, although testing becomes most effective when functional and immune assays are done in combination and multiple samples are taken. However, comparative and prospective studies have demonstrated that functional tests are more specific than enzyme immunoassays, are better at detecting the clinically significant HIT antibodies, and are more helpful in the diagnosis of HIT.
Monitoring
Because the drop in platelet count is a primary way of recognising HIT, routine monitoring of the platelet count is recommended for most patients receiving heparin treatment. The American College of Chest Physicians, in collaboration with the College of American Pathologists, have published a guideline15 in relation to monitoring of platelet count in patients at high risk for HIT. These recommendations are summarised in table 2. British guidelines29 recommend a baseline platelet count before initiating heparin treatment in all patients to allow estimation of relative changes. In higher risk patients, such as individuals receiving unfractionated heparin at therapeutic doses, the platelet count should be checked at least every other day from day 4 to 14 of treatment (or until heparin is stopped, whichever is sooner). In lower risk surgical, medical and obstetric patients, monitoring should be at least on every second to fourth day between days 4 and 14 while on heparin treatment. Obstetric patients receiving prophylactic doses of LMWH do not need routine platelet monitoring.
Table 2 Consensus guideline for platelet count monitoring for heparin induced thrombocytopenia15.
| Population | Example | Monitoring guideline* |
|---|---|---|
| Recent heparin exposure | Patients starting UFH or LMWH and who received UFH within the previous 100 days; patients whose heparin exposure history is unknown | Obtain baseline platelet count and repeat platelet count within 24 h of starting heparin |
| Acute, systemic reactions after intravenous UFH bolus | Patients with acute inflammatory, cardiorespiratory, neurological, or other unusual symptoms and signs within 30 min after an intravenous UFH bolus | Obtain platelet count immediately to compare with recent prior platelet counts |
| Risk of HIT >1%† | Patients receiving UFH at therapeutic doses | Monitor at least every 2 days until day 14 of treatment or until UFH is stopped, whichever comes first |
| Postoperative patients receiving UFH antithrombotic prophylaxis | Monitor at least every 2 days between postoperative days 4 and 14† or until UFH is stopped, whichever comes first | |
| Risk of HIT 0.1–1%† | Medical/obstetric patients receiving prophylactic‐dose UFH, or LMWH after first receiving UFH; postoperative patients receiving prophylactic dose LMWH, or intravascular catheter UFH flushes | Monitor every 2 or 3 days from days 4 to 14† or until UFH is stopped, whichever comes first, when practical |
| Risk of HIT <0.1%† | Medical/obstetric patients receiving only LMWH; medical patients receiving catheter UFH flushes | As clinically indicated (no routine monitoring) |
HIT, heparin induced thrombocytopenia; LMWH, low molecular weight heparin; UFH, unfractionated heparin.
*As recommended by College of American Pathologists. Adapted with permission from Warkentin TE, Greinacher A. Heparin‐induced thrombocytopenia: recognition, treatment, and prevention. Chest 2004;126:311S–37S.
†Risk stratification is based on the overall incidence of HIT in different patient population.
Treatment
The “4 T's” scoring system can be used as a guide to identify patients who are at high, intermediate or low risk of developing HIT. In our opinion, heparin should immediately be discontinued both in high risk and intermediate risk patients—although an alternative anticoagulant should also be initiated in the former but should only be considered in the latter after laboratory confirmation. Low risk patients only need continued platelet monitoring.
General principles
When HIT is suspected clinically, the following measures should be taken:
Immediate cessation of all formulations of heparin is mandatory including heparin flushes, heparin coated catheters, heparinised dialysate and any other sources
Send blood samples for laboratory confirmation
Initiate alternative anticoagulation. The duration of treatment is not well defined; however, it should be continued for at least 2–3 months to prevent recurrence of thrombosis
Monitor carefully for thrombotic event
Monitor platelet count till recovery
Warfarin should not be used until the platelet count has recovered
Avoid prophylactic platelet transfusion because they may exacerbate the hypercoagulable state, leading to additional thrombosis; however, if the patient develops bleeding or is undergoing major surgical intervention, therapeutic platelet transfusion can be considered.
Conventional strategies
Discontinuation of heparin alone will neither stop continuing thrombin generation nor avoid subsequent thrombotic events. The risk ranges from 5–10% per day in the first few days,30 increasing to 40–50% over the next several days or weeks.31 LMWH cannot be used in patients with HIT because of the strong crossreactivity of the HIT antibody with the LMWH–PF4 complex.32 Warfarin is no longer recommended in the early stage of HIT because it can paradoxically worsen the thrombosis and cause venous limb gangrene and skin necrosis.15 The probable mechanism is an imbalance between the natural anticoagulant and procoagulant proteins associated with HIT‐related consumption, which are exacerbated during warfarin induction. If a patient is receiving coumarin treatment when diagnosed with HIT, vitamin K administration is recommended to reverse the coumarin effects.15 Prostacyclin analogues act as natural vasodilators and inhibit platelet aggregation, but there is no protection from thrombosis.
Alternative anticoagulants
Currently, three non‐heparin anticoagulants that do not crossreact with HIT antibodies are available for alternative anticoagulation in HIT. These include danaparoid33 and lepirudin34 which available for use in the UK, whereas argatroban is used in North America.35 These drugs are immediately active and are direct inhibitors of thrombin, but they also inhibit thrombin generation and are routinely monitored with the activated partial thromboplastin time (aPTT) or, at higher levels of anticoagulation, the activated clotting time (ACT) for argatroban, ecarin clotting time (ECT) for lepirudin, or anti‐Xa assays for danaparoid.
As in all scenarios where antithrombotic drugs are used, the benefit is partially offset by haemorrhagic complications. Estimated incidence, as reported in retrospective studies, of major bleeding is higher with lepirudin (13–19%) than argatroban (6–7%).21 When choosing an alternative anticoagulant, consideration should be given to its demonstrated efficacy and safety in the intended use, likely risks and benefits of treatment strategies, availability of the drug and methods for monitoring, and the patient's clinical status, including renal and hepatic function.
Lepirudin
Recombinant hirudin (lepirudin), originally produced from the medicinal leech, is a 65 amino acid peptide with a molecular weight of approximately 7000 Da. It is a direct, irreversible thrombin inhibitor, binding both free and clot‐bound thrombin. It has a half‐life of 60–90 min with renal excretion. The incidence of the combined end point of death, new thromboembolic complications and limb amputation is lower in HIT patients treated with lepirudin with34 or without36 thrombosis. Currently, the recommended15 initial dose for patients with thrombosis is 0.4 mg/kg followed by a 0.15 mg/kg/h infusion (table 3), adjusted to aPTT ratios of 1.5–2.5, corresponding to a lepirudin concentration of approximately 0.6–1.4 mg/l. A lower dose (0.1 mg/kg/h) without a bolus is recommended in HIT patients without thrombosis.36 The risk of major haemorrhage is directly related to the aPTT ratio, lepirudin concentrations and serum creatinine concentrations.
Table 3 Therapeutic dosing regimen for danaparoid and lepirudin29.
| Drug | Weight | IV bolus | IV infusion | Monitoring |
|---|---|---|---|---|
| Danaparoid | <60 kg | 1500 U | 400 U/h for first 4 h, 300 U/h for next 4 h, then 150–200 U/h | Anti‐Xa factor |
| 60–74 kg | 2250 U | Range: 0.5–0.8 U/ml | ||
| 75–90 kg | 3000 U | |||
| >90 kg | 3750 U | |||
| Lepirudin | Maximum 100 kg | 0.4 mg/kg | Start at 0.15 mg/kg/h and titrate for target aPTT | aPTT 1.5–2.5 |
| Plasma concentration: 0.6–1.4 mg/l |
aPTT, activated partial thromboplastin time, IV, intravenous.
Lepirudin should be used with caution and in a reduced dosage in patients with serum creatinine values >1.6 mg/dl (141.4 μmol/l); it is contraindicated in patients on haemodialysis or with acute renal failure.37 Approximately 50% of patients may develop anti‐hirudin antibodies that bind the drug to form lepirudin–antibody complexes, too large for renal excretion, resulting in prolongation of the half‐life, increased plasma lepirudin concentrations and the need to reduce dose.38 Anaphylaxis, including anaphylactic death, occurs in an estimated 0.15% of patients on first exposure and in 0.2% of patients upon re‐exposure.39 Omitting the bolus dose may reduce the severity of anaphylaxis and non‐hirudin anticoagulants should be considered for use in patients with previous lepirudin exposure.39
Danaparoid
Danaparoid is a mixture of heparin, dermatan, and chondroitin sulfates and exerts its anticoagulant effects predominantly by inhibiting factor Xa and to a much lesser degree by inhibiting thrombin. It exhibits in vitro crossreactivity to HIT sera in about 10–50% of cases,40 but in vivo crossreactivity is rare although well described41 and has been associated with unfortunate treatment failures.42 Danaparoid has a long half‐life, near 100% bioavailability, and is cleared renally.
Danaparoid is approved by the US Food and Drug Administration (FDA) for venous thrombosis prophylaxis after orthopaedic surgery but not for the treatment of HIT, even though it has the longest track record of currently available agents. In the European Union29 it is approved for use in two distinct regimens: a low dose (“prophylactic”) and a high dose (“therapeutic”) regimens. The recommended dose for thromboprophylaxis in HIT patients without thrombosis is 750 U, administered subcutaneously twice or three times daily. The recommended treatment of HIT patients with thrombosis is 1500–3750 U bolus (depending on body weight) followed by a 400 U/h infusion for 4 h, then a 300 U/h infusion for 4 h, then a 150–200 U/h infusion for at least 5 days (table 3), with a target of 0.5–0.8 anti‐factor Xa U/ml in plasma.
Danaparoid in a high dose regimen is equivalent to lepirudin in the treatment of HIT with or without thrombosis; reduction in the incidence of the combined end point of death, new thromboembolic complications and limb amputation is comparable with lepirudin.43 In Britain, the prophylactic dose is not recommended in the treatment of HIT.
Argatroban
Argatroban, a direct inhibitor of thrombin, is an arginine‐based synthetic anticoagulant that reversibly binds with the catalytic site of thrombin. Although the British guideline29 does not recommend argatroban, it is approved by the US FDA for prophylaxis or treatment of thrombosis35 and during coronary angioplasty44 in patients with HIT. The recommended initial dose is 2 µg/kg/min adjusted to achieve aPTTs 1.5–3 times the baseline value which provides adequate anticoagulation for 90% of patients.21
A major advantage of argatroban is that it is cleared by the liver and, therefore, can be used safely in patients with renal insufficiency. Argatroban also has the shortest half‐life among all alternative anticoagulants and can be discontinued quickly if invasive procedures are necessary or if bleeding is encountered. There is no evidence of antibody generation to argatroban on prolonged or repeated administration, and no anaphylactic deaths have been reported.
Risk of bleeding with direct thrombin inhibitors
Unlike any other anticoagulant treatment, bleeding is a major safety concern with direct thrombin inhibitors because no specific antidotes are available and protamine sulfate only negligibly neutralises danaparoid. Unintentional excessive anticoagulation associated with or without bleeding should be managed by stopping or reducing the dose of direct thrombin inhibitors. With direct thrombin inhibitors, anticoagulant effects decrease to baseline, typically within hours, in accordance with the drug's elimination half‐life and the patient's organ function. However, the half‐life of argatroban (39–51 min) and lepirudin (1.7 h) are increased in hepatic impairment45 and renal impairment,46 respectively. Because danaparoid has a long half‐life (up to 25 h), rapid reversal after drug discontinuation is not an option. Haemodialysis or haemofiltration can sometimes reduce concentrations of lepirudin,47 but dialytic clearance of argatroban by high‐flux membranes is clinically insignificant.48
Additional treatment considerations
Fondaparinux, a synthetic pentasaccharide (smaller than LMWH) that is structurally related to the anti‐thrombin binding site of heparin, is expected to be less likely to induce HIT. However, it is not yet recommended for the use in patients with HIT, although preliminary data suggest that administration of fondaparinux 2.5 mg for at least 5 days to patients with HIT is not associated with continued or recurrent thrombocytopenia and no thrombotic complications occurred.49
Melagatran, and its oral prodrug ximelagatran, are small molecules that bind only at the thrombin active sites; they had been under intensive investigation before being withdrawn by the manufacturer (AstraZeneca) due to multiple reports of severe liver injury attributed to the drug. Attractive features included predictable pharmacokinetics and bioavailability, allowing for fixed dosing and predictable anticoagulant response, no need for routine coagulation monitoring, and rapid onset and offset of action.6
Bivalirudin is a hirudin analogue that has been effective in trials of angioplasty in patients with HIT, but is not approved for treatment of HIT. It is cleared by a combination of renal mechanisms and proteolytic cleavage, which may offer a pharmacological benefit for anticoagulation of patients with comorbid hepatic and renal disease.
Aspirin is beneficial in vitro and may have some clinical benefit. Platelet glycoprotein IIb/IIIa inhibitors reduce thrombin generation indirectly and inhibit platelet aggregation. Prostacyclin analogues act as natural vasodilators and inhibit platelet aggregation. However, these agents lack direct anticoagulant effects and do not inhibit Fc receptor‐mediated activation of platelets by HIT antibody.50 Hence, these agents should not be used as frontline treatment in HIT.
Surgical thromboembolectomy or systemic or local thrombolysis, as adjunctive treatment to alternative parenteral anticoagulation, may be appropriate for selected patients with large vessel arterial thromboembolism or severe pulmonary embolism. Plasmapheresis and use of intravenous immunoglobulin has been helpful in anecdotal reports, but whether it is of additional benefit to current treatments is unclear. Inferior vena cava filters have led to worsening of thrombotic problems in a number of cases. Their use in HIT has not been studied, and most physicians avoid their use.
Warfarin and HIT
If warfarin treatment is indicated for an underlying medical condition or HIT‐associated deep vein thrombosis (DVT), it must be delayed until adequate alternative parenteral anticoagulation has been provided and platelet counts have recovered substantially15 (to at least 100×109/l or preferably 150×109/l). Warfarin should be started at the expected maintenance dose (maximum 5 mg) and not at a loading dose. Parenteral anticoagulation should be overlapped with warfarin for minimum of 5 days until a target international normalised ratio (INR) range has been achieved for at least 2 days.15
Direct thrombin inhibitors as a class prolong the prothrombin time and INR,51 the extent of which depends on the drug and its concentration; this effect is particularly pronounced with argatroban,46 making the transition to warfarin more difficult. If warfarin has already been started when HIT is recognised, reversal of warfarin with vitamin K is recommended15 for two reasons: to minimise the risk of microvascular thrombosis and consequent skin necrosis, and to prevent under dosing of direct thrombin inhibitors.
Key points
Heparin induced thrombocytopenia (HIT) is an immune‐mediated event that can have severe life‐ and limb‐threatening complications.
Despite thrombocytopenia, bleeding is rare; rather, HIT is strongly associated with thromboembolic complications.
When a diagnosis of HIT is suspected immediate cessation of all forms of heparin, including unfractionated heparin (UFH), low molecular weight heparin (LMWH) and heparin flushes, is imperative.
Treatment of HIT should be initiated based on clinical suspicion and must never be delayed pending laboratory confirmation of HIT.
A direct thrombin inhibitor, such as lepirudin, danaparoid or argatroban, is considered the agent of choice for treatment of HIT.
Warfarin should not be used until the platelet count has recovered.
Anticoagulation in patients with a history of HIT
Following the development of hypersensitivity to a drug, it is generally accepted that further exposure should be avoided if possible. Anticoagulation in patients with a history of HIT depends upon the type of surgery, whether it can be delayed or not, whether anticoagulation is desired for therapeutic or prophylactic reasons, and whether patient is HIT antibodies positive or negative. HIT antibodies are transient with a median time to disappearance of 50–80 days.29 Because the consequences of recurrent HIT may be devastating, in the vast majority of cases (excluding cardiac and vascular) where a patient with previous HIT requires a period of anticoagulation or anticoagulant prophylaxis, it is acceptable to use an anticoagulant alternative to UFH or LMWH.15
Cardiovascular surgery
The haemostasis and thrombosis task force of the British Haematology Society recommends29 that in all patients with a history of HIT, who lack detectable HIT antibodies and require cardiac or vascular surgery, care must be taken to minimise heparin exposure, using it only during surgery and administering alternative anticoagulation, when needed, before and after surgery.
In patients with acute or active HIT or with a history of HIT and lingering HIT antibodies, cardiovascular surgery should be delayed until HIT is fully resolved and antibodies are undetectable by a sensitive assay. If delay is impossible or the urgency of the situation precludes assessment of HIT antibody status in a patient with a history of HIT, alternative anticoagulation should preferably be used intraoperatively.15,29
Limited experience exists with lepirudin, argatroban, bivalirudin, and danaparoid, sometimes together with antiplatelet agents, in this setting. It is emphasised that safe, effective doses of alternative anticoagulants during cardiovascular surgery have not been validated in clinical trials.
Percutaneous coronary intervention
The American College of Chest Physicians suggest the use of an alternative anticoagulant in patients with or at risk for HIT who is undergoing percutaneous coronary intervention (PCI). Argatroban is the only alternative anticoagulant approved in the USA for the purpose, and its safety and efficacy in this setting is validated.44 Bivalirudin is FDA approved as an anticoagulant in patients undergoing percutaneous transluminal coronary angioplasty,52 but experience with lepirudin in patients with HIT undergoing PCI is limited.50 The British Society of Haematology does not provide any guidance in this setting.
Haemodialysis
Only anecdotal reports are available on anticoagulation for dialysis‐dependant patients with HIT. Suggested alternatives include saline solution flushing, citrate, danaparoid, lepirudin and argatroban. Table 4 shows the regimens for danaparoid and lepirudin for alternate day haemodialysis in patients who have previously had HIT.29
Table 4 Regimens for danaparoid and lepirudin for alternate day haemodialysis in patients who have previously had HIT29.
| Drugs | IV bolus | Monitoring | |
|---|---|---|---|
| Danaparoid | 3750 (2500) U* before first and second dialyses; 3000 U before third dialysis; then according to pre‐dialysis anti‐Xa level | Anti‐Xa 0.5–0.8 U/ml | |
| <0.3 | 3000 (2000) U | ||
| 0.3–0.35 | 2500 (1500) U | ||
| 0.35–0.4 | 2000 (1500) U | ||
| >0.4 | 0 U | ||
| Lepirudin | Lepirudin 80–150 μg/kg before dialysis | aPTT 2.0–2.5 | |
aPTT, activated partial thromboplastin time, IV, intravenous.
*For danaparoid use doses in parentheses for patients <55 kg.
Paediatrics
Reports on the treatment of paediatric HIT patients are generally anecdotal.53 A study is ongoing in the USA to evaluate argatroban anticoagulation, including its pharmacokinetics, in paediatric patients in whom heparin use is problematic.
Prevention
The incidence of HIT can be reduced by:
Limiting courses of heparin to <5 days, if possible.
Using LMWH in place of heparin for thromboprophylaxis in high‐risk postoperative patients.
Porcine UFH is associated with lower incidence of HIT then bovine UFH.
In general, monitoring the platelet count at least every other day between days 4 and 14 of heparin exposure or until heparin is discontinued.
Taking care not to automatically initiate heparin if a patient is re‐admitted for a thrombotic event; the medical record must be thoroughly reviewed for use of heparin on a previous admission (within the past 100 days). Unfortunately, in some cases, the previous heparin exposure may not be recorded in the medical record (for example, heparin flushes).
Orders for use of heparin flushes and heparinised normal saline must be written and signed by a physician and use of heparin flushes and heparinised normal saline must be documented in the patient's medical record.
Patient information and record keeping
The diagnosis of HIT should be clearly recorded in the patient's notes and marked as a serious allergy. The condition should be clearly explained to the patient and an information leaflet may be helpful in this respect. The patient should be issued with an antibody card.
Multiple choice questions (true (T)/false (F); answers after the references)
1. Regarding incidence of HIT:
Incidence is highest in cardiac surgery patients
Occurs more frequently with porcine than bovine heparin
Incidence is not influenced by the dose, type and route of heparin administration
Severe thrombocytopenia (platelet count <15×109/l) in HIT is very common.
Obstetric patients are at low risk for HIT
2. Clinical features of HIT include:
Fall in platelet count typically starting within hours after initiation of heparin
Thrombocytopenia is severe and frequently results in bleeding
Thrombosis in HIT is associated with a mortality of approximately 20–30%
Obstetric patients are particularly at risk of developing thrombotic complications
Females are more likely to suffer thrombotic stroke as an outcome of their HIT
3. Pathology of HIT:
The principal antigen is a complex of heparin and platelet factor 4 (PF4)
The principle antibody generated is an IgA antibody
UFH antibody does not crossreact with LMWH antibody
All patients who develop HIT antibodies will subsequently develop clinical syndrome of HIT
HIT antibodies begin to disappear in 4–10 days after cessation of heparin treatment
4. Treatment strategies for HIT:
UFH should be immediately substituted with LMWH
Argatroban is the most commonly used alternative anticoagulant in the UK
Warfarin is contraindicated in HIT
Danaparoid exerts its anticoagulant effects predominantly by inhibiting factor Xa
Anaphylaxis is a recognised complication associated with argatroban
5. Regarding HIT:
Routine monitoring of the platelet count is recommended for most patients receiving heparin treatment
Thrombocytopenia should be treated with transfusion of washed platelets
Excessive anticoagulation associated with danaparoid can completely be reversed with protamine sulfate
In patients with a history of HIT, heparin can safely be used intraoperatively during cardiac surgery
Prostacyclin analogues are safe anticoagulants for haemodialysis in patients with HIT
Abbreviations
ACT - activated clotting time (ACT)
aPTT - activated partial thromboplastin time
DVT - deep vein thrombosis
ECT - ecarin clotting time
FDA - Food and Drug Administration
HIPA - heparin‐induced platelet aggregation, HIT, heparin‐induced thrombocytopenia
INR - international normalised ratio
LMWH - low molecular weight heparins
PCI - percutaneous coronary intervention
PF4 - platelet factor 4
SRA - serotonin release assay
UFH - unfractionated heparins
ANSWERS
(A) T (B) F (C) F (D) F (E) T
(A) T (B) F (C) T (D) F (E) T
(A) T (B) F (C) F (D) F (E) F
(A) F (B) F (C) T (D) T (E) F
(A) T (B) F (C) F (D) T (E) F
Footnotes
Competing interests: None declared.
References
- 1.Chong B H. Heparin‐induced thrombocytopenia. J Thromb Haemost 200311471–1478. [DOI] [PubMed] [Google Scholar]
- 2.Campbell K R, Mahaffey K W, Lewis B E.et al Bivalirudin in patients with heparin‐induced thrombocytopenia undergoing percutaneous coronary intervention. J Invasive Cardiol 200012(suppl F)14F–19F. [PubMed] [Google Scholar]
- 3.Strauss R, Wehler M, Mehler K.et al Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med 2002301765–1771. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M. Heparin induced thrombocytopenia: an update. Thrombosis Journal 2005314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice L. Heparin‐induced thrombocytopenia: myths and misconceptions. Arch Intern Med 20041641961–1964. [DOI] [PubMed] [Google Scholar]
- 6.Katzung B G.Basic and clinical pharmacology, 9th ed. McGraw Hill 2004545–549.
- 7.Reilly R F. The pathophysiology of immune‐mediated heparin‐induced thrombocytopenia. Semin Dial 20031654–60. [DOI] [PubMed] [Google Scholar]
- 8.Amiral J, Bridey F, Wolf M.et al Antibodies to macromolecular platelet factor 4–heparin complexes in heparin‐induced thrombocytopenia: a study of 44 cases. Thromb Haemost 19957321–28. [PubMed] [Google Scholar]
- 9.Kelton J G, Smith J W, Warkentin T E.et al Immunoglobulin G from patients with heparin‐induced thrombocytopenia binds to a complex of heparin and platelet factor 4. Blood 1994833232–3239. [PubMed] [Google Scholar]
- 10.Denomme G A, Warkentin T E, Horsewood P.et alJ Lab Clin Med 1997130278–284. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson L E, Lubenow N, Blumentritt C.et alPharmacogenetics 200313253–258. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin T E, Levine M N, Hirsh J.et al Heparin‐induced thrombocytopenia in patients treated with low‐molecular‐weight heparin or unfractionated heparin. N Engl J Med 19953321330–1335. [DOI] [PubMed] [Google Scholar]
- 13.Kelton JG: Heparin‐induced thrombocytopenia: an overview Blood Rev. 2002;16:77–80. doi: 10.1054/blre.2001.0189. [DOI] [PubMed] [Google Scholar]
- 14.Nand S, Wong W, Yuen B.et al Heparin‐induced thrombocytopenia with thrombosis: incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol 19975612–16. [DOI] [PubMed] [Google Scholar]
- 15.Warkentin T E, Greinacher A. Heparin‐induced thrombocytopenia: recognition, treatment, and prevention. Chest 2004126311S–37S. [DOI] [PubMed] [Google Scholar]
- 16.Warkentin T E, Sheppard J I, Horsewood P.et al Impact of the patient population on the risk for heparin‐induced thrombocytopenia. Blood 2000961703–1708. [PubMed] [Google Scholar]
- 17.Andreescu A C, Possidente C, Hsieh M.et al Evaluation of a pharmacy‐based surveillance program for heparin‐induced thrombocytopenia. Pharmacotherapy 200220974–980. [DOI] [PubMed] [Google Scholar]
- 18.Warkentin T E. Heparin‐induced thrombocytopenia: a clinicopathologic syndrome. Thromb Haemost 199982439–447. [PubMed] [Google Scholar]
- 19.Lubenow N, Kempf R, Eichner A.et al Heparin‐induced thrombocytopenia: temporal pattern of thrombocytopenia in relation to initial use or reexposure to heparin. Chest 200212237–42. [DOI] [PubMed] [Google Scholar]
- 20.Warkentin T E, Kelton J G. Delayed‐onset heparin‐induced thrombocytopenia and thrombosis. Ann Intern Med 2001135502–506. [DOI] [PubMed] [Google Scholar]
- 21.Jang I K, Hursting M J. When heparins promote thrombosis: review of heparin‐induced thrombocytopenia. Circulation 20051112671–2683. [DOI] [PubMed] [Google Scholar]
- 22.Meyer‐Lindenberg A, Quenzel E M, Bierhoff E.et al Fatal cerebral venous sinus thrombosis in heparin‐induced thrombotic thrombocytopenia. Eur Neuro 199737191–192. [DOI] [PubMed] [Google Scholar]
- 23.Boshkov L K, Warkentin T E, Hayward C P.et al Heparin‐induced thrombocytopenia and thrombosis: clinical and laboratory studies. Br J Haematol 199384322–328. [DOI] [PubMed] [Google Scholar]
- 24.Kelton J G, Hursting M J, Lewis B E. The predictors of clinical outcome in patients with heparin‐induced thrombocytopenia. J Thromb Haemost 20031(supp1)OC020 [abstract]. [DOI] [PubMed] [Google Scholar]
- 25.Opatrny L, Warner M N. Risk of thrombosis in patients with malignancy and heparin‐induced thrombocytopenia. Am J Hematol 200476240–244. [DOI] [PubMed] [Google Scholar]
- 26.LaMonte M P, Brown P M, Hursting M J. Stroke in patients with heparin‐induced thrombocytopenia and the effect of argatroban therapy. Crit Care Med 200432976–980. [DOI] [PubMed] [Google Scholar]
- 27.Warkentin T E, Aird W C, Rand J H. Platelet‐endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology (American Society of Hematology Education Program) 2003497–519. [DOI] [PubMed]
- 28.Francis J, Drexler A. Frequency of heparin–platelet factor 4 antibodies in patients presenting to the emergency room with symptoms of thrombosis. J Thomb Haemost 20031(suppl 1)P1147 [abstract] [Google Scholar]
- 29.Keeling D, Davidson S, Watson H. Guideline: the management of heparin‐induced thrombocytopenia. Br J Haematol 2006133259–269. [DOI] [PubMed] [Google Scholar]
- 30.Greinacher A, Eichler P, Lubenow N.et al Heparin‐induced thrombocytopenia with thromboembolic complications: meta‐analysis of 2 prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood 200096846–851. [PubMed] [Google Scholar]
- 31.Klein H G, Bell W R. Disseminated intravascular coagulation during heparin therapy. Ann Intern Med 197480477–481. [DOI] [PubMed] [Google Scholar]
- 32.Keeling D M, Richards E M, Baglin T P. Platelet aggregation in response to four low molecular weight heparins and the heparinoid ORG 10172 in patients with heparin‐induced thrombocytopenia. Br J Haematol 199486425–426. [DOI] [PubMed] [Google Scholar]
- 33.Chong B H, Gallus A S, Cade J F, for the Australian HIT Study Group Prospective randomised open‐label comparison of danaparoid with dextran 70 in the treatment of heparin‐induced thrombocytopaenia with thrombosis: a clinical outcome study. Thromb Haemost 2001861170–1175. [PubMed] [Google Scholar]
- 34.Greinacher A, Eichler P, Lubenow N.et al Heparin‐induced thrombocytopenia with thromboembolic complications: meta‐analysis of 2 prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood 200096846–851. [PubMed] [Google Scholar]
- 35.Lewis B E, Wallis D E, Berkowitz S D , for the ARG‐911 Study Investigators. Argatroban anticoagulant therapy in patients with heparin‐induced thrombocytopenia. Circulation 20011031838–1843. [DOI] [PubMed] [Google Scholar]
- 36.Lubenow N, Eichler P, Lietz T.et al Lepirudin for prophylaxis of thrombosis in patients with acute isolated heparin‐induced thrombocytopenia: an analysis of three prospective studies. Blood 20041043072–3077. [DOI] [PubMed] [Google Scholar]
- 37.Vanholder R, Camez A, Veys N.et al Pharmacokinetics of recombinant hirudin in hemodialyzed end‐stage renal failure patients. Thromb Haemost 199777650–655. [PubMed] [Google Scholar]
- 38.Eichler P, Friesen H J, Lubenow N.et al Antihirudin antibodies in patients with heparin‐induced thrombocytopenia treated with lepirudin: incidence, effects on aPTT and clinical relevance. Blood 2000962373–2378. [PubMed] [Google Scholar]
- 39.Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin‐induced thrombocytopenia. Circulation 20031082062–2065. [DOI] [PubMed] [Google Scholar]
- 40.Chong B H, Magnani H N. Danaparoid for the treatment of heparin‐induced thrombocytopenia: an overview. In: Warketin TE, Greinacher A, eds. Heparin‐induced thrombocytopenia, 3rd ed. New York: Marcel Dekker, 2004371–396.
- 41.Keng T B, Chong B H. Heparin‐induced thrombocytopenia and thrombosis syndrome: in vivo cross‐reactivity with danaparoid and successful treatment with r‐Hirudin. Br J Haematol 2001114394–396. [DOI] [PubMed] [Google Scholar]
- 42.Kodityal S, Manhas A H, Udden M.et al Danaparoid for heparin‐induced thrombocytopenia: an analysis of treatment failures. Eur J Haematol 200371109–113. [DOI] [PubMed] [Google Scholar]
- 43.Farner B, Eichler P, Kroll H.et al A comparison of danaparoid and lepirudin in heparin‐induced thrombocytopenia. Thromb Haemost 200185950–957. [PubMed] [Google Scholar]
- 44.Lewis B E, Matthai W H, Cohen M.et al Argatroban anticoagulation during percutaneous coronary intervention in patients with heparin‐induced thrombocytopenia. Cath Cardiovasc Interv 200257177–184. [DOI] [PubMed] [Google Scholar]
- 45.Swan S K, Hursting M J. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy 200020318–329. [DOI] [PubMed] [Google Scholar]
- 46.Fox I, Dawson A, Loynds P.et al Anticoagulant activity of hirulog, a direct thrombin inhibitor, in humans. Thromb Haemost 199369157–163. [PubMed] [Google Scholar]
- 47.Fischer K G. Hirudin in renal insufficiency. Semin Thromb Hemost 200228467–482. [DOI] [PubMed] [Google Scholar]
- 48.Murray P M, Reddy B V, Grossman E J.et al A prospective comparison of three argatroban treatment regimens during in end‐stage renal disease. Kidney Int 2004662446–2453. [DOI] [PubMed] [Google Scholar]
- 49.Bradner J, Hallisey R K, Kuter D J. Fondaparinux in the treatment of heparin‐induced thrombocytopenia. Blood 2004104492a [Google Scholar]
- 50.Pinto D S, Sperling R T, Tu R M.et al Combination platelet glycoprotein IIb/IIIa receptor and lepirudin administration during percutaneous coronary intervention in patients with heparin‐induced thrombocytopenia. Cath Cardiovasc Interv 20035865–68. [DOI] [PubMed] [Google Scholar]
- 51.Gosselin R C, Dager W E, King J H.et al Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and argatroban, on prothrombin time and INR values. Am J Clin Pathol 2004121593–599. [DOI] [PubMed] [Google Scholar]
- 52.Bittl J A, Chaitman B R, Feit F.et al Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: final report reanalysis of the Bivalirudin Angioplasty Study. Am Heart J 2001142952–959. [DOI] [PubMed] [Google Scholar]
- 53.Alsoufi B, Boshkov L K, Kirby A.et al Heparin‐induced thrombocytopenia (HIT) in pediatric cardiac surgery: an emerging cause of morbidity and mortality. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual 20047155–171. [DOI] [PubMed] [Google Scholar]