Abstract
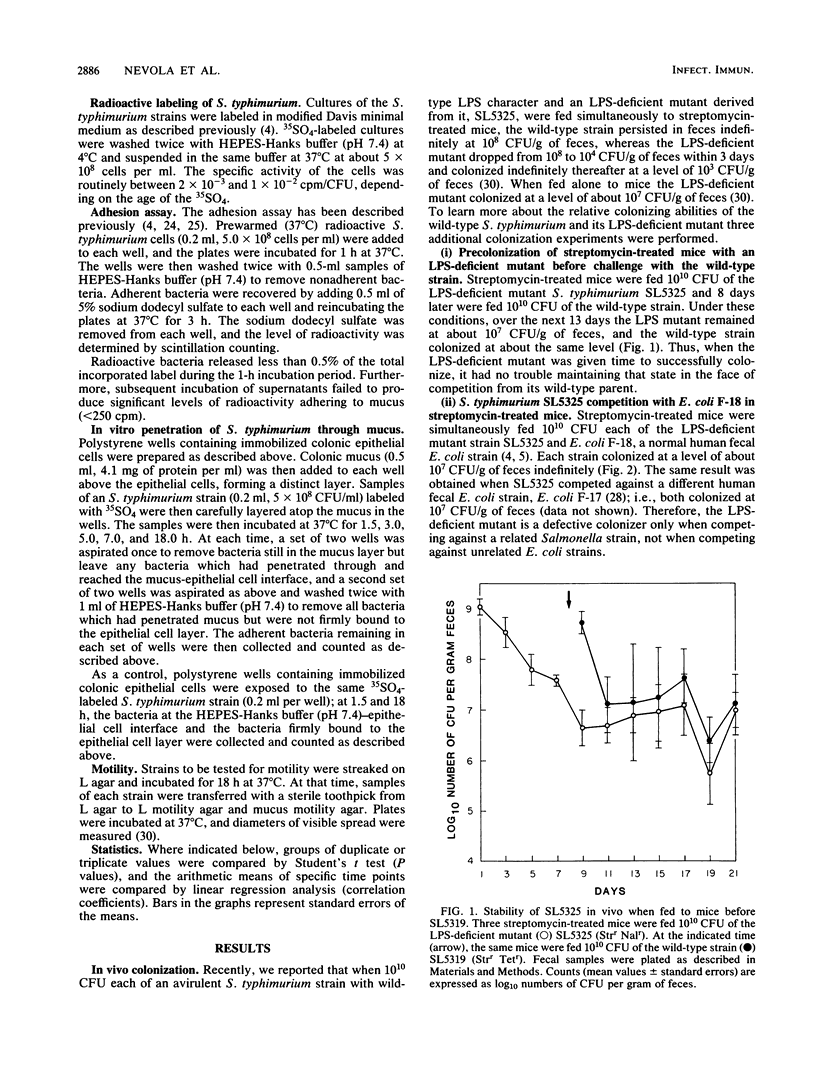
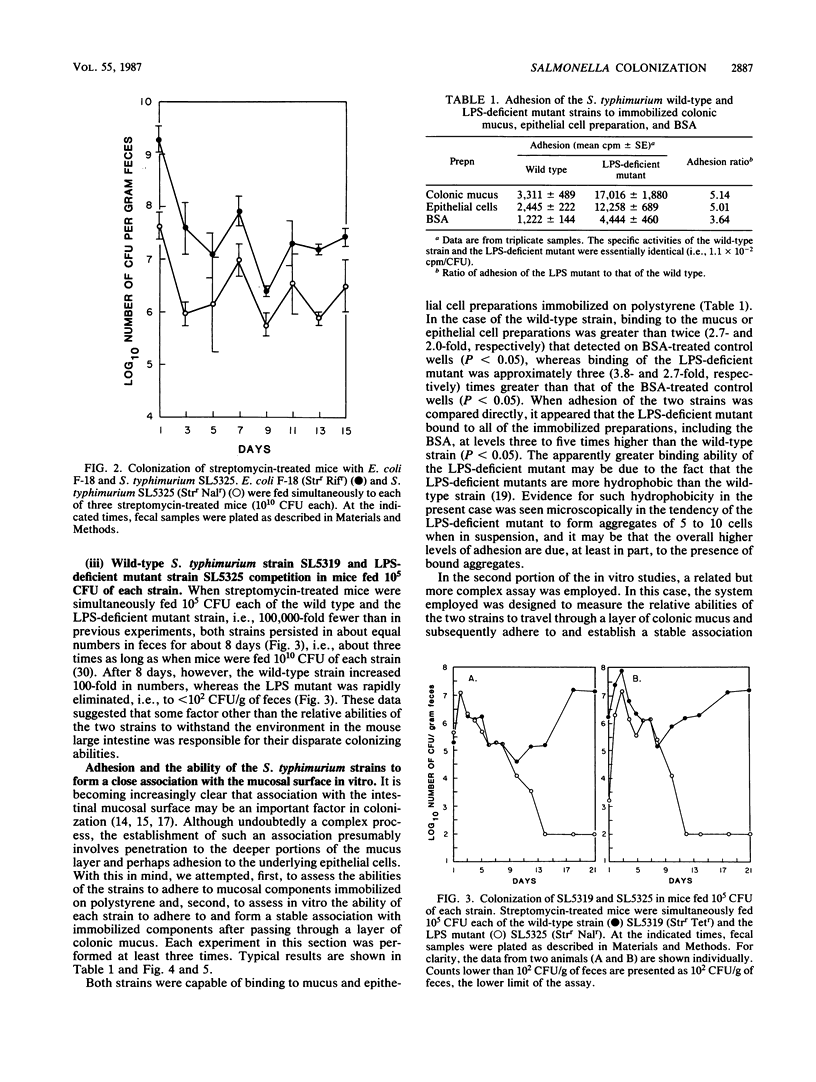
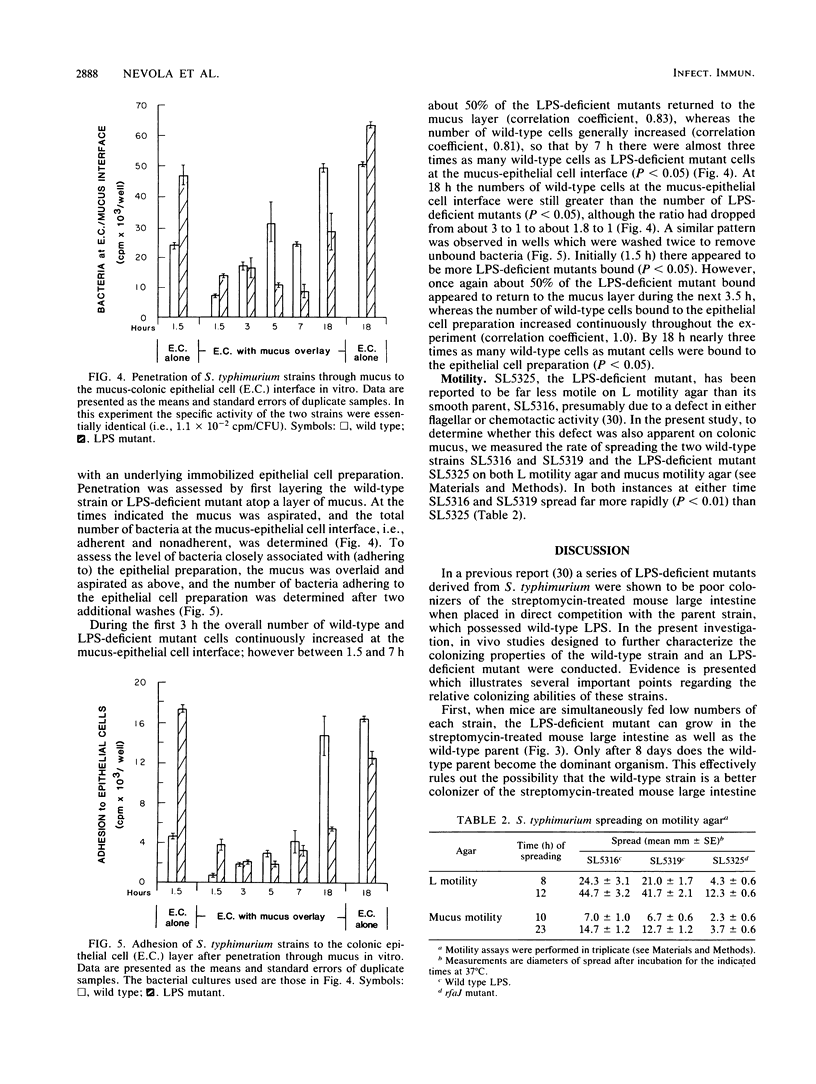
The relative abilities of an avirulent Salmonella typhimurium strain with wild-type lipopolysaccharide (LPS) character, SL5319, and a nearly isogenic LPS-deficient mutant, SL5325, to colonize the large intestines of streptomycin-treated CD-1 mice in vivo and to penetrate colonic mucus in vitro were studied. Previously it had been shown that, when fed simultaneously to streptomycin-treated mice (approximately 10(10) CFU each), the S. typhimurium strain with wild-type LPS colonized at 10(8) CFU/g of feces indefinitely, whereas the LPS-deficient mutant dropped within 3 days to a level of only 10(4) CFU/g of feces. In the present investigation, when SL5325 was allowed to colonize for 8 days before feeding mice SL5319 or when it was fed to mice simultaneously with an Escherichia coli strain of human fecal origin (10(10) CFU each), both strains colonized indefinitely at 10(7) CFU/g of feces. Moreover, when the wild-type and LPS-deficient mutant strains were fed to mice simultaneously in low numbers (approximately 10(5) CFU each) the strains survived equally well in the large intestines for 8 days, after which the LPS-deficient mutant was eliminated (less than 10(2) CFU/g of feces), whereas the wild-type colonized at a level of 10(7) CFU/g of feces. In addition although both strains were able to adhere to mucus and epithelial cell preparations in vitro, the wild-type strain was shown to have greater motility and chemotactic activity on CD-1 mouse colonic mucus in vitro and to more rapidly penetrate and form a stable association with immobilized colonic mucosal components in vitro. Based on these data, we suggest that the ability of an S. typhimurium strain to colonize the streptomycin-treated mouse large intestine may, in part, depend on its ability to penetrate deeply into the mucus layer on the intestinal wall and subsequently, through growth, colonize the mucosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. II. FACTORS RESPONSIBLE FOR ITS LOSS FOLLOWING STREPTOMYCIN TREATMENT. J Exp Med. 1964 Nov 1;120:817–828. doi: 10.1084/jem.120.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinari G., Hale T. L., Washington O., Formal S. B. Effect of guinea pig or monkey colonic mucus on Shigella aggregation and invasion of HeLa cells by Shigella flexneri 1b and 2a. Infect Immun. 1986 Mar;51(3):975–978. doi: 10.1128/iai.51.3.975-978.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Alfredsson G. A., Barker R., Old D. C. A new biotyping scheme for Salmonella typhimurium and its phylogenetic significance. J Med Microbiol. 1975 Feb;8(1):149–166. doi: 10.1099/00222615-8-1-149. [DOI] [PubMed] [Google Scholar]
- FRETER R. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J Exp Med. 1956 Sep 1;104(3):411–418. doi: 10.1084/jem.104.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRETER R. The fatal enteric cholera infection in the guinea pig, achieved by inhibition of normal enteric flora. J Infect Dis. 1955 Jul-Aug;97(1):57–65. doi: 10.1093/infdis/97.1.57. [DOI] [PubMed] [Google Scholar]
- Forstner G. G. (1-14C)glucosamine incorporation by subcellular fractions of small intestinal mucosa. Identification by precursor labeling of three functionally distinct glycoprotein classes. J Biol Chem. 1970 Jul 25;245(14):3584–3592. [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Fekete J., Vickerman M. M., Carey K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983 Feb;39(2):686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Mechanisms of association of bacteria with mucosal surfaces. Ciba Found Symp. 1981;80:36–55. doi: 10.1002/9780470720639.ch4. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968 Feb;54(2):210–217. [PubMed] [Google Scholar]
- Ikari N. S., Kenton D. M., Young V. M. Interaction in the germfree mouse intestine of colicinogenic and colicin-sensitive microorganisms. Proc Soc Exp Biol Med. 1969 Apr;130(4):1280–1284. doi: 10.3181/00379727-130-33773. [DOI] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhal M. L., Cohen P. S., Laux D. C. Altered colonizing ability for mouse large intestine of a surface mutant of a human faecal isolate of Escherichia coli. J Gen Microbiol. 1983 May;129(5):1549–1558. doi: 10.1099/00221287-129-5-1549. [DOI] [PubMed] [Google Scholar]
- Nevola J. J., Stocker B. A., Laux D. C., Cohen P. S. Colonization of the mouse intestine by an avirulent Salmonella typhimurium strain and its lipopolysaccharide-defective mutants. Infect Immun. 1985 Oct;50(1):152–159. doi: 10.1128/iai.50.1.152-159.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977 Aug;60(2):272–277. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- QUASTLER H., SHERMAN F. G. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res. 1959 Jun;17(3):420–438. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- Que J. U., Casey S. W., Hentges D. J. Factors responsible for increased susceptibility of mice to intestinal colonization after treatment with streptomycin. Infect Immun. 1986 Jul;53(1):116–123. doi: 10.1128/iai.53.1.116-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Rubulis A., Rubert M., Faloon W. W. Cholesterol lowering, fecal bile acid, and sterol changes during neomycin and colchicine. Am J Clin Nutr. 1970 Sep;23(9):1251–1259. doi: 10.1093/ajcn/23.9.1251. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Yano S., Slomiany B. L., Glass G. B. Lipid composition of the gastric mucous barrier in the rat. J Biol Chem. 1978 Jun 10;253(11):3785–3791. [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wold J. K., Khan R., Midtvedt T. Intestinal glycoproteins of germfree rats. Chemical composition of intestinal and fecal mucus from germfree rats fed a chemically defined diet. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):525–530. [PubMed] [Google Scholar]



